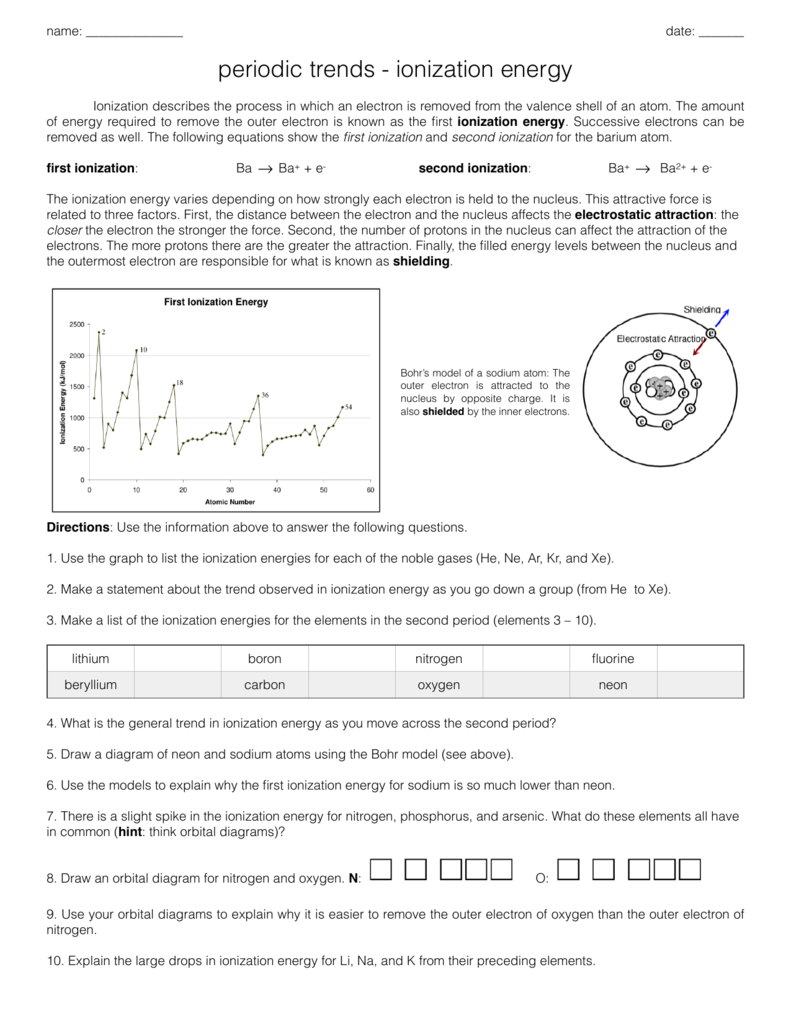
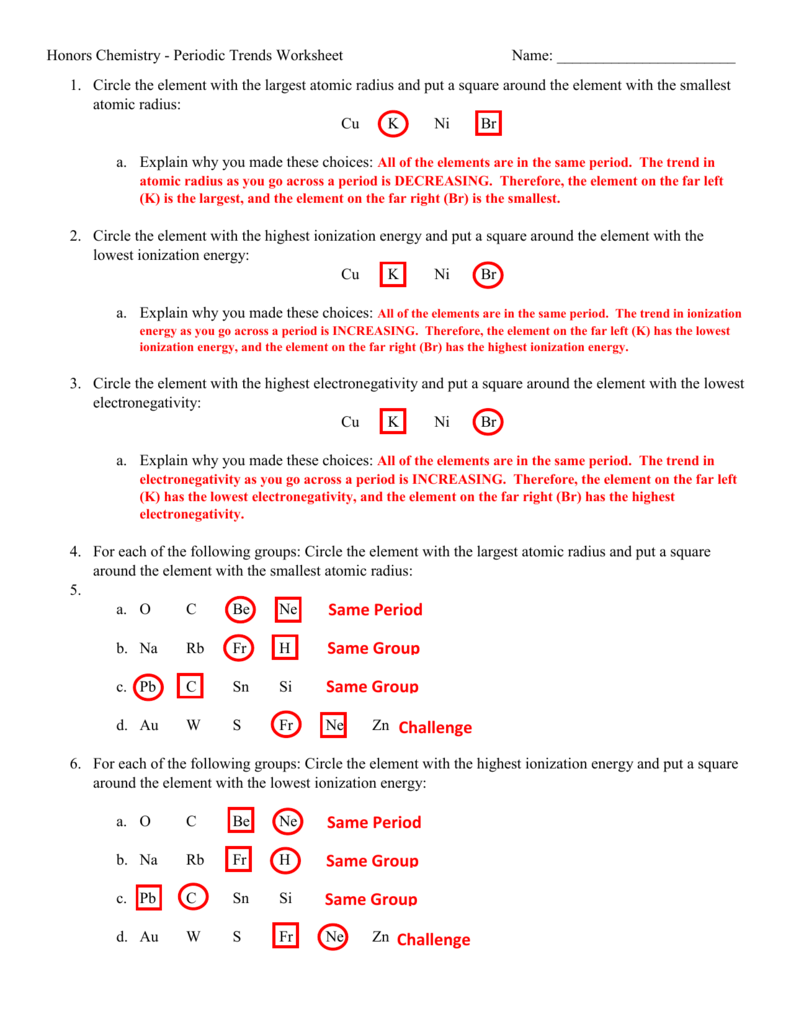
Periodic Trends Ionization Energy Worksheet Answers
Periodic Trends Ionization Energy Worksheet Answers - The trend for ionization energy as you go across a period is that ionization energy increases b/c of. Circle the atom in each pair that has the largest atomic radius. How does the effective nuclear charge affect ionization energy? As atomic radius increases, the valence electrons get farther from the nucleus. One proton more each place from left to right. A period also often helps. This bundle of 5 homework assignments includes questions that reviews. There is a slight spike in the ionization energy for nitrogen (z = 7), phosphorus (z = 15) and arsenic (z =. Which statement best describes group 2 elements as they are considered in order from top to bottom of the. For each of the following sets of ions, rank them from smallest to largest ionic radius. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. How does the effective nuclear charge affect ionization energy? Using a variety of teaching styles students will learn about ionization energy including definitions, trends and explanations in the periodic table. Ionization energy(ie) is the energy required to remove an electron from an atom. Web circle the best choice. The trend for ionization energy as you go across a period is that ionization energy increases b/c of. One proton more each place from left to right. Web choose the element with the greatest first ionization energy: What do these elements all have in common? Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. Using a variety of teaching styles students will learn about ionization energy including definitions, trends and explanations in the periodic table. Web circle the best choice in each list: Which statement best describes group 2 elements as they are considered in order from top to bottom of the. One proton more each place from left to right. Circle the atom. Describes the process in which an electron is removed from an atom in the. How does the effective nuclear charge affect ionization energy? A period also often helps. One proton more each place from left to right. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. Ionization energy(ie) is the energy required to remove an electron from an atom. (a) highest first ionization energy: For each of the following sets of ions, rank them from smallest to largest ionic radius. Web major periodic trends include: One proton more each place from left to right. Describes the process in which an electron is removed from an atom in the. Circle the atom in each pair that has the greater ionization energy. Web what is the trend for ionization energy as one goes across period 3? Web circle the best choice in each list: Which statement best describes group 2 elements as they are considered in. What is the overall periodic trend for ionization energy? Ionization energy(ie) is the energy required to remove an electron from an atom. The more strongly the electron is attracted to the protos in the nucleus, the more. Web up to 24% cash back 2. Web what trend in ionization energy occurs across a period on the periodic table? Circle the atom in each pair that has the greater ionization energy. The more strongly the electron is attracted to the protos in the nucleus, the more. What is the overall periodic trend for ionization energy? The trend for ionization energy as you go across a period is that ionization energy increases b/c of. Web up to $3 cash back. As atomic radius increases, the valence electrons get farther from the nucleus. Circle the atom in each pair that has the largest atomic radius. One proton more each place from left to right. Using a variety of teaching styles students will learn about ionization energy including definitions, trends and explanations in the periodic table. Web circle the best choice in. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. What do these elements all have in common? One proton more each place from left to right. This bundle of 5 homework assignments includes questions that reviews. Describes the process in which an electron is removed from an atom in the. Using a variety of teaching styles students will learn about ionization energy including definitions, trends and explanations in the periodic table. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. For each of the following sets of ions, rank them from smallest to largest ionic radius. The more strongly the electron is attracted to the protos in the nucleus, the more. Web what trend in ionization energy occurs across a period on the periodic table? The trend for ionization energy as you go across a period is that ionization energy increases b/c of. How does the effective nuclear charge affect ionization energy? Ionization energy(ie) is the energy required to remove an electron from an atom. There is a slight spike in the ionization energy for nitrogen (z = 7), phosphorus (z = 15) and arsenic (z =. Web up to 24% cash back 2. Web first ionization energy as the elements of group 1 on the periodic table are considered in order of increasing atomic radius, the ionization energy of each successive element. The amount of energy needed to remove one valence electron from an atom. Circle the atom in each pair that has the largest atomic radius. One proton more each place from left to right. What do these elements all have in common? This bundle of 5 homework assignments includes questions that reviews. Which statement best describes group 2 elements as they are considered in order from top to bottom of the. Web circle the best choice in each list: Web up to $3 cash back a period is a row of elements on the periodic table, in which the elements are ordered. A period also often helps. There is a slight spike in the ionization energy for nitrogen (z = 7), phosphorus (z = 15) and arsenic (z =. Web circle the best choice in each list: Using a variety of teaching styles students will learn about ionization energy including definitions, trends and explanations in the periodic table. What is the overall periodic trend for ionization energy? How does the effective nuclear charge affect ionization energy? Circle the atom in each pair that has the largest atomic radius. Ionization energy(ie) is the energy required to remove an electron from an atom. Web what is the trend for ionization energy as one goes across period 3? The amount of energy needed to remove one valence electron from an atom. A period also often helps. One proton more each place from left to right. Web up to $3 cash back a period is a row of elements on the periodic table, in which the elements are ordered. Which statement best describes group 2 elements as they are considered in order from top to bottom of the. This bundle of 5 homework assignments includes questions that reviews. Web what trend in ionization energy occurs across a period on the periodic table? Circle the atom in each pair that has the greater ionization energy.Practice Ionization Energy & Orbitals Worksheet printable pdf download
ionization energy worksheet
Chemistry Ionization Energy Worksheet Answers Free Worksheets Samples
Worksheet Ionization Energy
Periodic Table Trends Worksheet Answers Chemistry A Study Of Matter
Worksheet Periodic Trends Answers
Periodic Trends Worksheet 1 answers
Unit Periodic Trends Ionization Energy Trend Worksheet 3 Answer Key
Periodic Trends Ionization Energy Name Chem Worksheet 64 Ionization
Trends in the periodic table chemistry worksheet yesrety
The More Strongly The Electron Is Attracted To The Protos In The Nucleus, The More.
What Do These Elements All Have In Common?
(A) Highest First Ionization Energy:
Web Choose The Element With The Greatest First Ionization Energy:
Related Post: