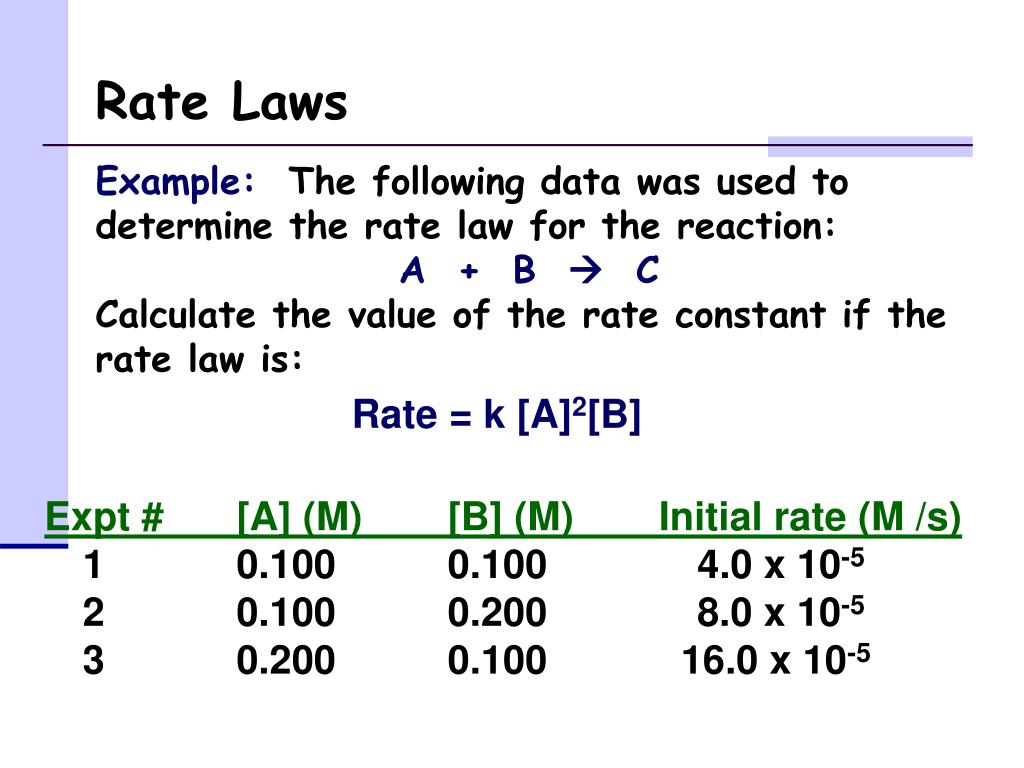
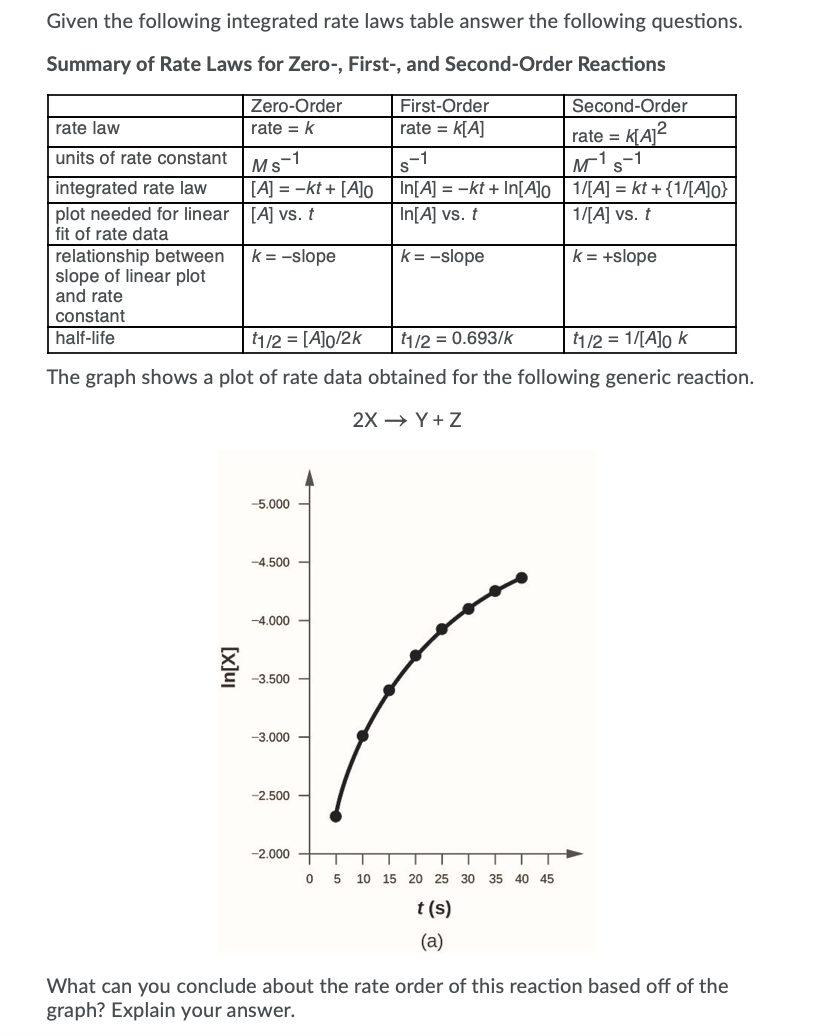
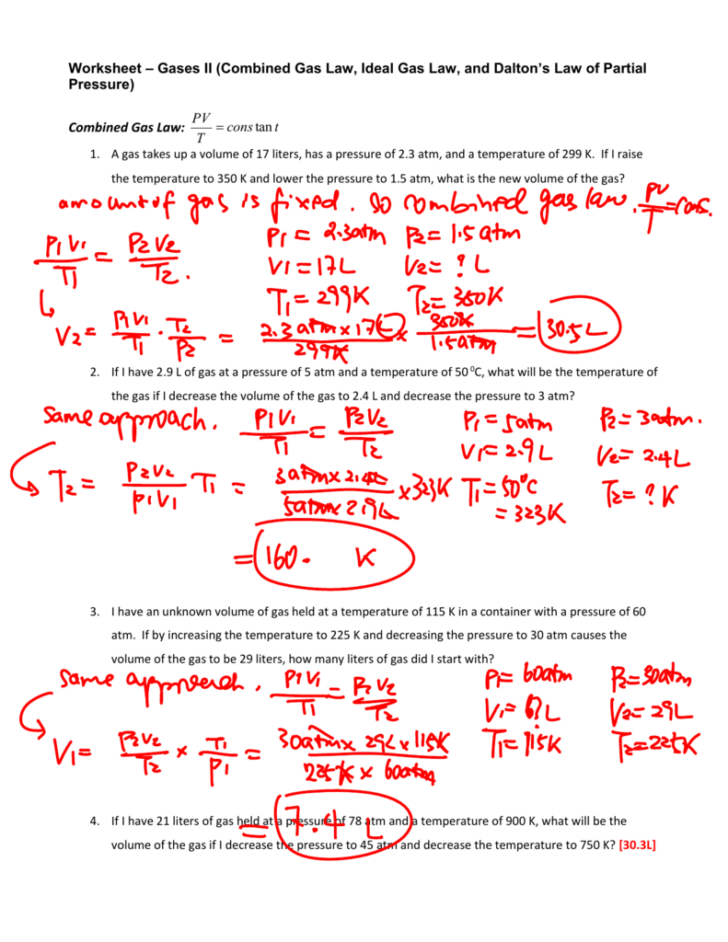
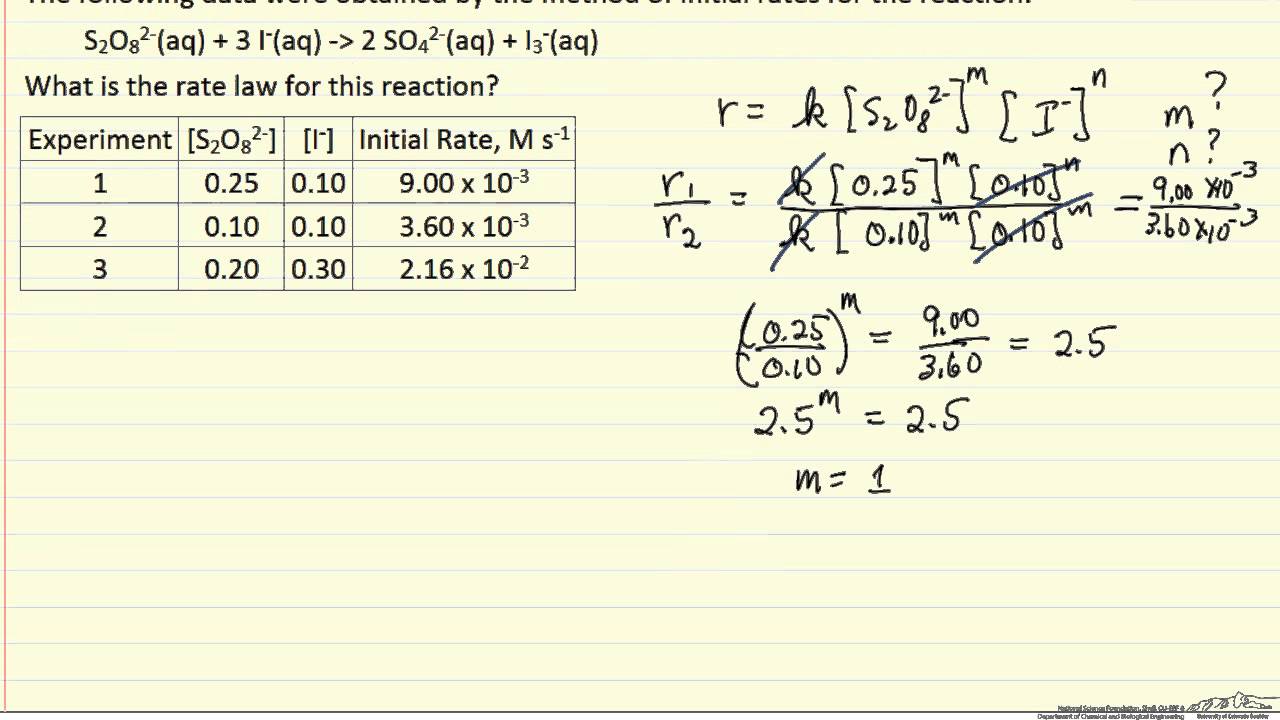
Rate Law Worksheet With Answers
Rate Law Worksheet With Answers - Web answers to discussion worksheet 6 1. Topics to know for the quiz include reaction order and the rate law equation. Given reaction rate data for: (a) rate = k[n 2o][o], (b) rate = k[o 2]2, (c) rate = k[clco][cl 2] 2. Web the rate law will have the form: Web to find [h2o2] at 1955 seconds we use the value of k we just found in the integrated rate law. Web you will receive your score and answers at the end. Worksheet on rate law expressions. Determine which values you intend to use. What is the rate law? Describes the dependence of the (forward) rate on the concentrations of reactants. For the reaction 2 no (g) + cl2(g) →. We will take [h2o2]0 = 0.500 m at 0.0 seconds. (and only for an elementary reaction) , the rate. Web up to 24% cash back rate laws worksheet i. Determine the order of each reactant and the overall reaction order. Web answers to discussion worksheet 6 1. Describes the dependence of the (forward) rate on the concentrations of reactants. Worksheet on rate law expressions. Yes, since the rate law is written for the slowest step of the reaction,. [h2o2] at 1955 sec = 0.0193 m. From 1 → 2 [h2o2] doubles and rate. Web up to 24% cash back rate laws worksheet i. Using the experimental data provided, determine the order of reaction with respect to each reactant, write the rate law, determine the. Web we can use integrated rate laws with experimental data that consist of time. Web we can use integrated rate laws with experimental data that consist of time and concentration information to determine the order and rate constant of a reaction. Describes the dependence of the (forward) rate on the concentrations of reactants. (and only for an elementary reaction) , the rate. Determine which values you intend to use. Web a) rate = k. Describes the dependence of the (forward) rate on the concentrations of reactants. Web to find [h2o2] at 1955 seconds we use the value of k we just found in the integrated rate law. (and only for an elementary reaction) , the rate. We will take [h2o2]0 = 0.500 m at 0.0 seconds. For the reaction 2 no (g) + cl2(g). Web the rate expression or rate law expression is the relation between reaction rate and the concentrations of reactants given by a mathematical equation. (and only for an elementary reaction) , the rate. Twice as fast as c increases; Web up to $3 cash back a) rate = k[no2]2 b) rate = k c) rate = k[h2][br2]1/2 d) rate =. Web up to $3 cash back a) rate = k[no2]2 b) rate = k c) rate = k[h2][br2]1/2 d) rate = k[no]2[o2] a) second order b) zero order c) 3/2 order d) third order 5. Decrease by 0.00050 m as measured in an “iodine clock” reaction at 23°c. Describes the dependence of the (forward) rate on the concentrations of reactants.. (and only for an elementary reaction) , the rate. Web the rate law will have the form: [h2o2] at 1955 sec = 0.0193 m. Decrease by 0.00050 m as measured in an “iodine clock” reaction at 23°c. Yes, since the rate law is written for the slowest step of the reaction,. Web you will receive your score and answers at the end. Web up to 24% cash back rate laws worksheet i. What is the rate law? Compare rates using the chart, remember that when comparing rates for element a, the values for element b must be the same. Given reaction rate data for: We will take [h2o2]0 = 0.500 m at 0.0 seconds. Given reaction rate data for: Web we can use integrated rate laws with experimental data that consist of time and concentration information to determine the order and rate constant of a reaction. Determine which values you intend to use. Compare rates using the chart, remember that when comparing rates for. Web up to 24% cash back rate laws worksheet i. Compare rates using the chart, remember that when comparing rates for element a, the values for element b must be the same. Web you will receive your score and answers at the end. Web use the following data to answer the questions below: Given reaction rate data for: Web up to $3 cash back a) rate = k[no2]2 b) rate = k c) rate = k[h2][br2]1/2 d) rate = k[no]2[o2] a) second order b) zero order c) 3/2 order d) third order 5. Web to find [h2o2] at 1955 seconds we use the value of k we just found in the integrated rate law. Web a) rate = k [no2]2 b) rate = k c) rate = k [h2] [br2]1/2 d) rate = k [no]2[o a) second order b) zero order c) 3/2 order d) third order 5. [h2o2] at 1955 sec = 0.0193 m. Using the experimental data provided, determine the order of reaction with respect to each reactant, write the rate law, determine the. We will take [h2o2]0 = 0.500 m at 0.0 seconds. What is the rate law? (a) rate = k[n 2o][o], (b) rate = k[o 2]2, (c) rate = k[clco][cl 2] 2. For the reaction 2 no (g) + cl2(g) →. Twice as fast as c increases; Worksheet on rate law expressions. Topics to know for the quiz include reaction order and the rate law equation. Web we can use integrated rate laws with experimental data that consist of time and concentration information to determine the order and rate constant of a reaction. (and only for an elementary reaction) , the rate. Decrease by 0.00050 m as measured in an “iodine clock” reaction at 23°c. Determine the order of each reactant and the overall reaction order. Web the rate expression or rate law expression is the relation between reaction rate and the concentrations of reactants given by a mathematical equation. Decrease by 0.00050 m as measured in an “iodine clock” reaction at 23°c. Web the rate law will have the form: Describes the dependence of the (forward) rate on the concentrations of reactants. Web answers to discussion worksheet 6 1. Web up to $3 cash back a) rate = k[no2]2 b) rate = k c) rate = k[h2][br2]1/2 d) rate = k[no]2[o2] a) second order b) zero order c) 3/2 order d) third order 5. Determine which values you intend to use. Web you will receive your score and answers at the end. Twice as fast as c increases; Web a) rate = k [no2]2 b) rate = k c) rate = k [h2] [br2]1/2 d) rate = k [no]2[o a) second order b) zero order c) 3/2 order d) third order 5. Web to find [h2o2] at 1955 seconds we use the value of k we just found in the integrated rate law. We will take [h2o2]0 = 0.500 m at 0.0 seconds. Given reaction rate data for: Yes, since the rate law is written for the slowest step of the reaction,. Compare rates using the chart, remember that when comparing rates for element a, the values for element b must be the same.√ 11 Boyle039s Law Worksheet Answers Simple Template Design
Rate Law Worksheet Answers Rate Law Worksheet 1. The rate of
Rate Laws Worksheet by Olivia Hunter Issuu
2 differential rate law worksheet
How To Determine Order Of Reaction From Rate Law
Solved Given the following integrated rate laws table answer
Ideal Gas Law Worksheet Answer Key —
How To Find Rate Law From Equation
Chapter 14 CHEMISTRY
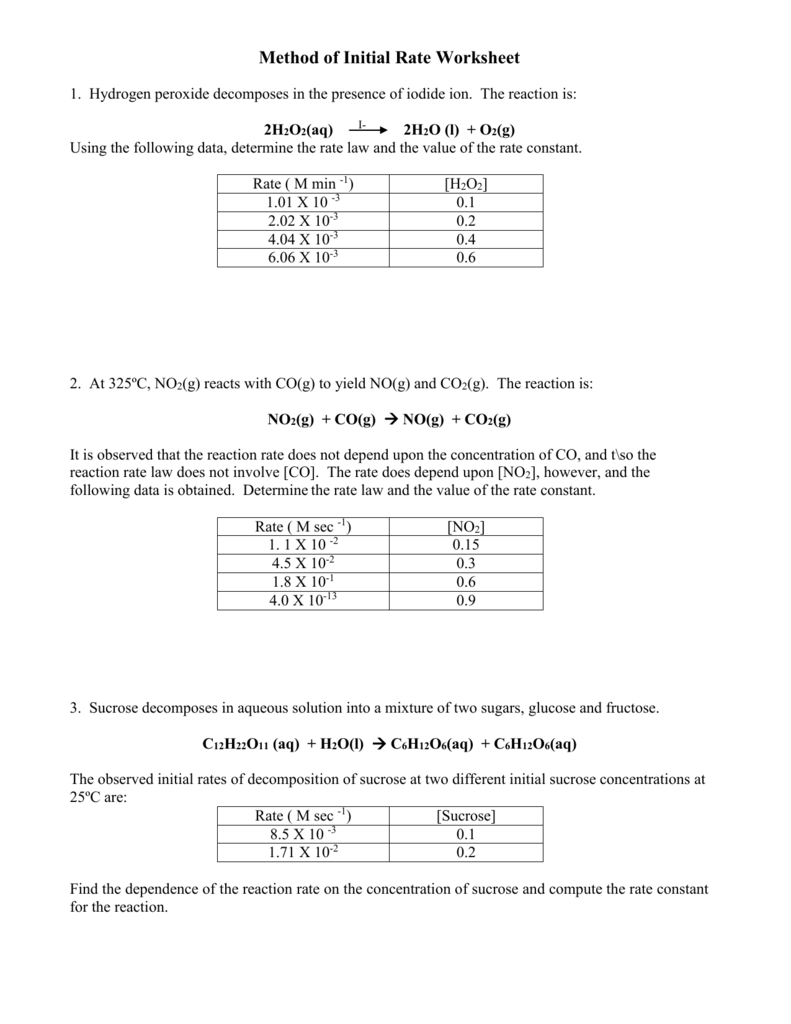
Method of Initial Rate Worksheet
What Is The Rate Law?
(A) Rate = K[N 2O][O], (B) Rate = K[O 2]2, (C) Rate = K[Clco][Cl 2] 2.
Web Use The Following Data To Answer The Questions Below:
[H2O2] At 1955 Sec = 0.0193 M.
Related Post: