Solubility Curve Worksheet 2
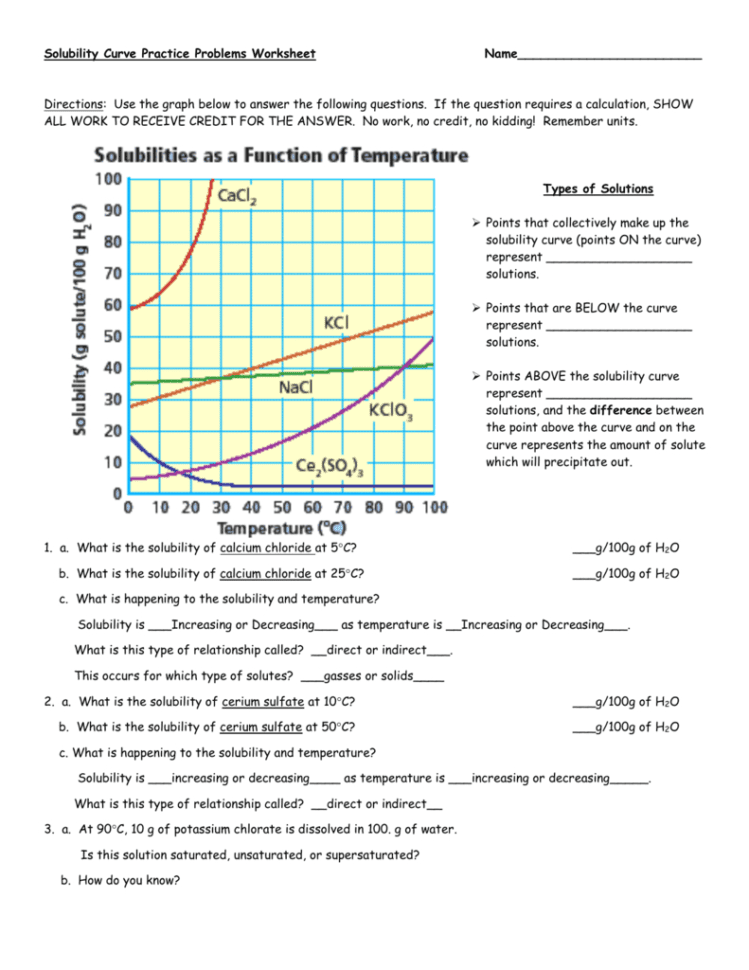
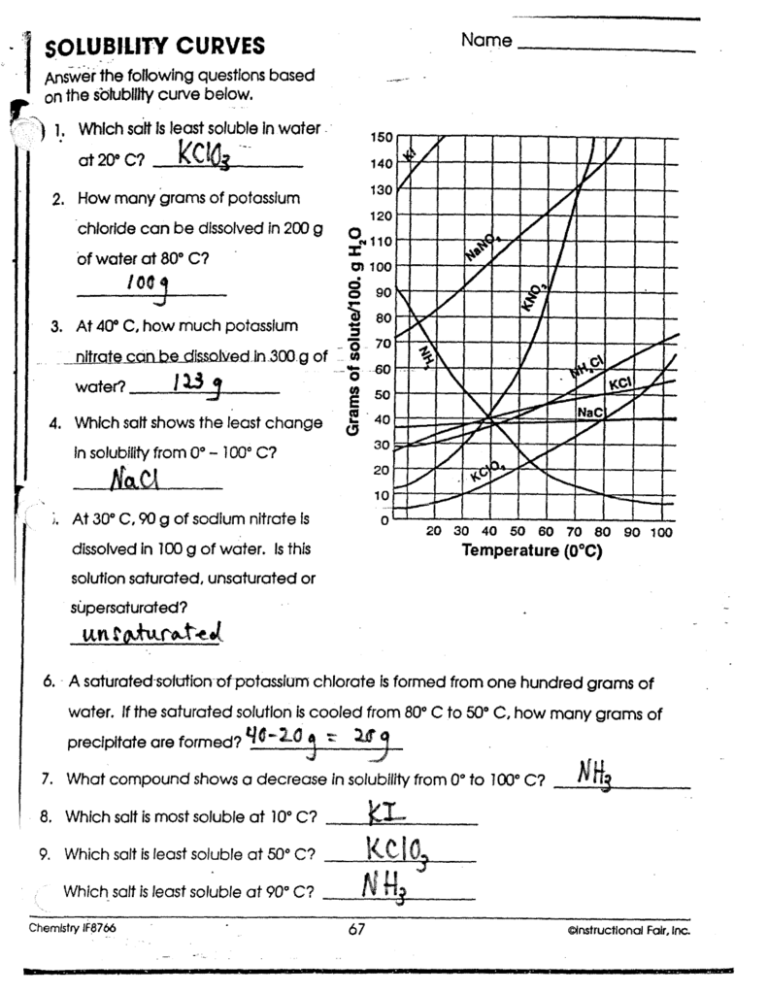
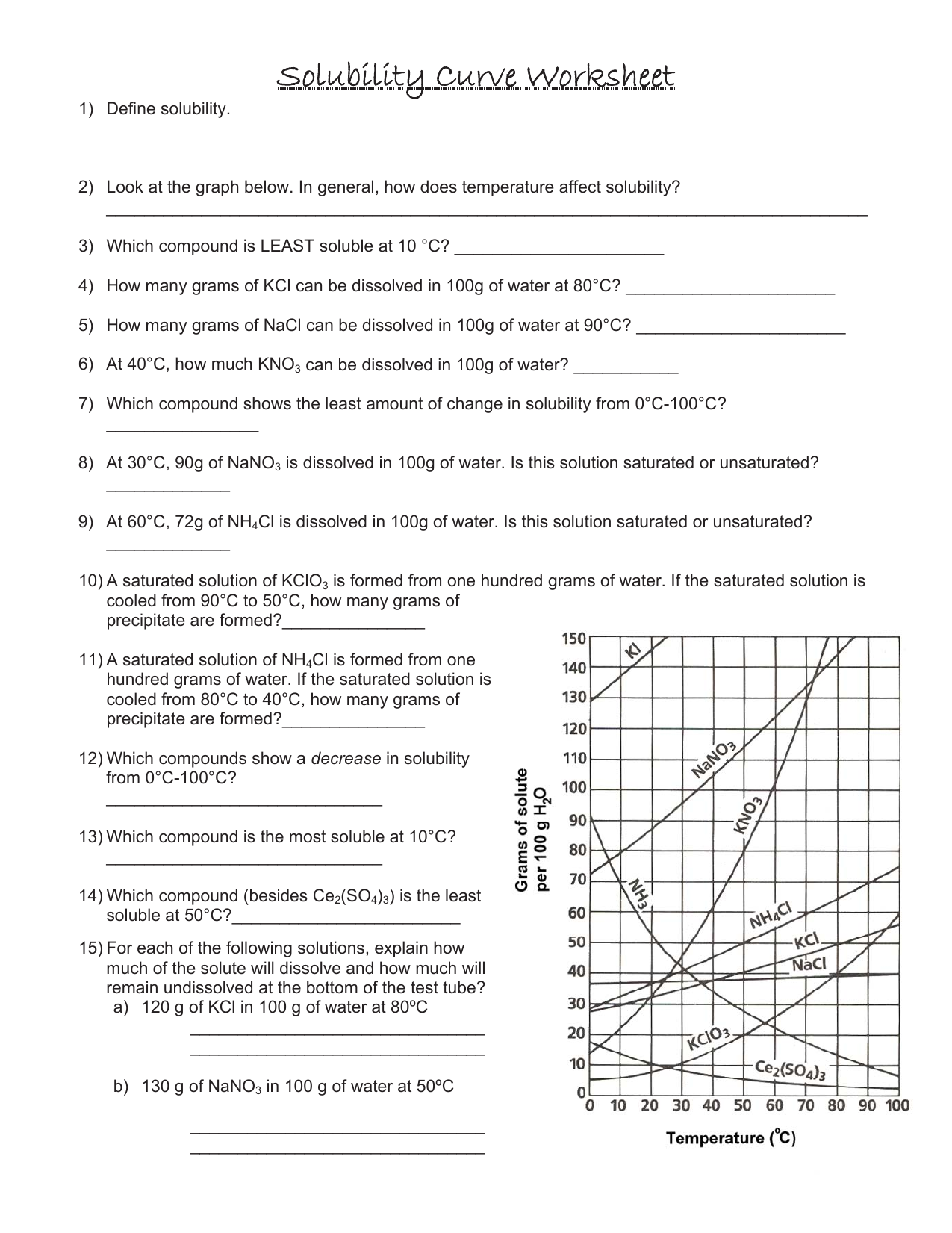
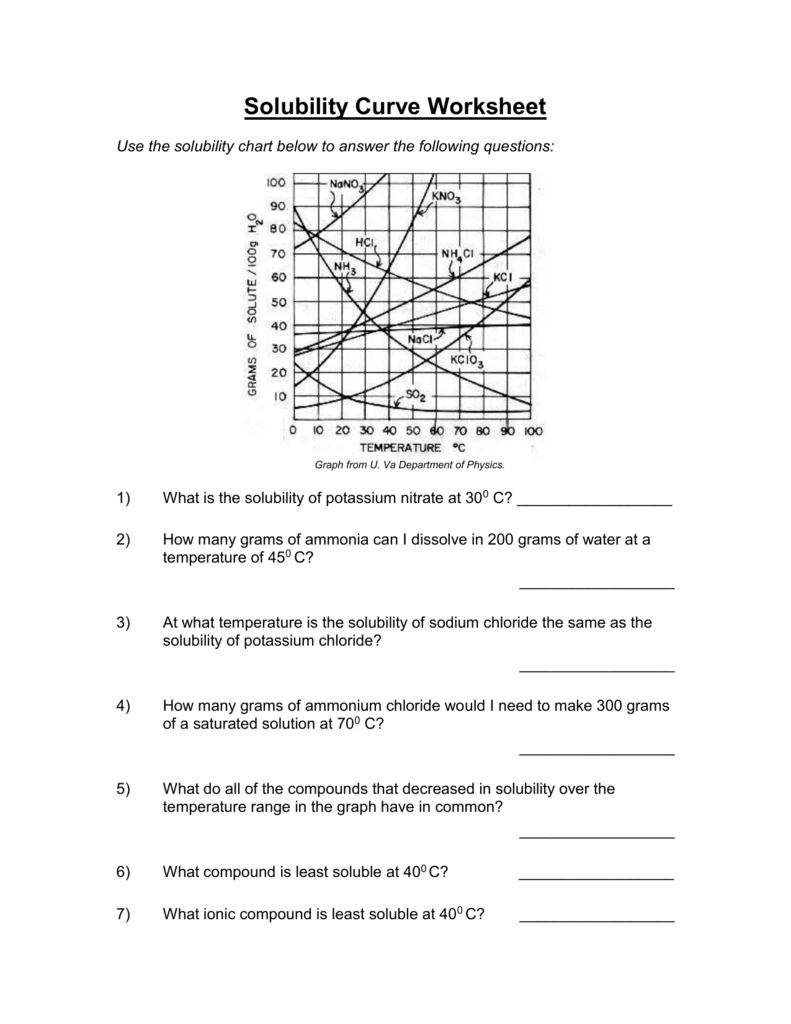
Solubility Curve Worksheet 2 - Soluble in water at 15°c. A detailed answer key is included.this product includes 16 problems covering:determining amount of solute dissolved given temperaturedetermining temperature given amount. Dissolving in ____________ grams of water. Of water at the following temperatures? At 90(c, you dissolved 10 g of kcl in 100. A measure of how much solute can dissolve in a given amount of solvent. Nh 4 cl at 90°c 72g / 100ml 4. Web use the solubility curves on the attached page to answer the following questions. List the substances whose solubility decreases as temperature increases. What are the customary units of solubility on solubility curves? At 90(c, you dissolved 10 g of kcl in 100. Nh 4 cl at 90°c 72g / 100ml 4. According to the graph, the solubility of any substance changes as _____ changes. The lesson covers the following objectives: Use the data table to construct a line graph. Kno3 at 70°c ____________ nacl at 100°c ____________ nh4cl at 90°c ____________ which of the. If all of the solute could be dissolved in 100 g of water at the given temperature, would the resulting solution be unsaturated, saturated, or supersaturated? Web use your solubility curve graphs provided to answer the following questions. What are the customary units of solubility. Nh 4 cl at 90°c solution: Of water at the following temperatures? At 90(c, you dissolved 10 g of kcl in 100. If all of the solute could be dissolved in 100 g of water at the given temperature, would the resulting solution be unsaturated, saturated, or supersaturated? What is the solubility of ce2(so4)3 at 50(c? Web we have not yet considered the solubility of a solute. Three substances is most soluble in water at 15°c. 60 g kcl at 70 °c _____ 2. Use the chemical agcl to describe solubility, molar solubility and solubility product. Degress celsius and grams of solute/100g of water 2. According to the graph, the solubility of any substance changes as _____ changes. Use your solubility curve graph provided to answer the following questions. If all of the solute could be dissolved in 100 g of water at the given temperature, would the resulting solution be unsaturated, saturated, or supersaturated? Use this resource as classwork, homework, extra practice, or examples. What is the solubility of ce2(so4)3 at 50(c? Is this solution saturated or. Web use the solubility curves on the attached page to answer the following questions. What is the solubility of kcl at 25(c? In general for solids, as. Web practice interpreting solubility curves and calculating various concentrations of solutions with this bundle of worksheets. Solubility is defined as the maximum mass of solute that can dissolve in a certain quantity of solvent at a specified temperature. Use the data table to construct a line graph. Degress celsius and grams of solute/100g of water 2. Three substances is most. A measure of how much solute can dissolve in a given amount of solvent. Three substances is most soluble in water at 15°c. Temperature increases, the solubility of the salt ___________________________. Is the solution saturated or unsaturated example: As the water temperature increases, does the amount of ammonium chloride. What are the customary units of solubility on solubility curves? According to the graph, the solubility of any substance changes as temperature changes. Web use the provided solubility graph to answer the following questions: In general for solids, as. What are the customary units of solubility on solubility curves? As the water temperature increases, does the amount of ammonium chloride. Is the solution saturated or unsaturated example: Soluble in water at 15°c. Below is a data table that shows the solubility of ammonium chloride (nh4cl) at various temperatures. Use the data table to construct a line graph. Use this resource as classwork, homework, extra practice, or examples with work shown for students in a distance learning setting. Use the data table to construct a line graph. What is the solubility of kcl at 25(c? What are the customary units of solubility on solubility curves? What are the customary units of solubility on solubility curves? Web solubility curve practice problems worksheet 1. Is this solution saturated or. Use the data table to construct a line graph. Web solubility curveworksheet use your solubility curve graph provided to answer the following questions. According to the graph, the solubility of any substance changes as _________ changes. Web what are the customary units of solubility on solubility curves? A measure of how much solute can dissolve in a given amount of solvent. According to the graph, the solubility of any substance changes as temperature changes. The amount of a substance that will dissolve in a given amount of another substance according to the graph, the solubility of any substance chang. At 90(c, you dissolved 10 g of kcl in 100. Web we have not yet considered the solubility of a solute. Use the chemical agcl to describe solubility, molar solubility and solubility product. Degress celsius and grams of solute/100g of water 2. List the substances whose solubility decreases as. Silver chloride has a larger ksp than silver carbonate ( ksp = 1.6 × 10 ‐ 10 and 8.1 × 10 ‐ 12 respectively). Use the chemical agcl to describe solubility, molar solubility and solubility product. Of water at the following temperatures? What are the customary units of solubility on solubility curves? Nh 4 cl at 90°c solution: Web what are the customary units of solubility on solubility curves? Web solubility curve practice problems worksheet 1. Below is a data table that shows the solubility of ammonium chloride (nh4cl) at various temperatures. A detailed answer key is included.this product includes 16 problems covering:determining amount of solute dissolved given temperaturedetermining temperature given amount. The amount of a substance that will dissolve in a given amount of another substance according to the graph, the solubility of any substance chang. As the name implies this is a worksheet to allow the student addtitional practice and time interpreting solubility curves (graphs). Web practice interpreting solubility curves and calculating various concentrations of solutions with this bundle of worksheets. Degress celsius and grams of solute/100g of water. List the substances whose solubility decreases as. How many grams of ammonium chloride will dissolve at a temperature of. Use the data table to construct a line graph. Nh 4 cl at 90°c 72g / 100ml 4.Solubility Curve Worksheet 2 Answer Key
Solubility Curve Practice Problems Worksheet Part 2 Answers
Solubility Curve Worksheets 2 Answer Key
solubility curves
Solubility Curve Worksheets 2 Answer Key
solubility curve worksheet 2 answers
Solubility Curve Worksheets 2 Answer Key
Solubility Curve Worksheet
20++ Solubility Worksheet Answer Key
Solubility Curve Worksheet With Answers
List The Substances Whose Solubility Decreases As Temperature Increases.
What Is The Solubility Of Kcl At 5(C?
Use This Resource As Classwork, Homework, Extra Practice, Or Examples With Work Shown For Students In A Distance Learning Setting.
Is This Solution Saturated Or.
Related Post: