Solubility Rules Worksheet
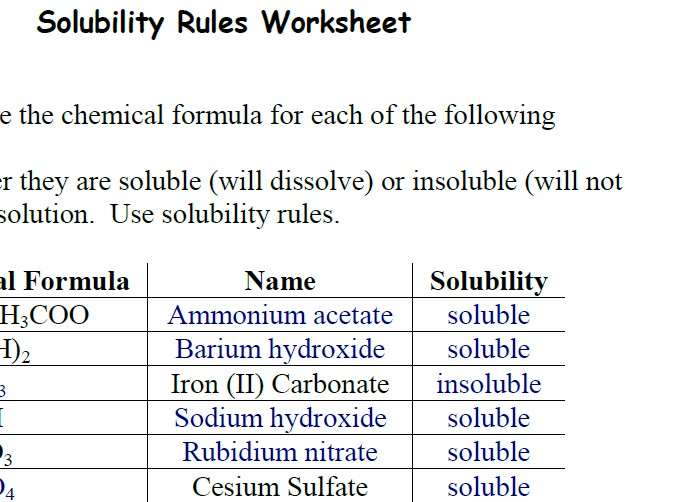
Solubility Rules Worksheet - Rbno3 rubidium nitrate yes 6. Web solubility rules worksheet classify each of the substances as being soluble or insoluble in water. Which of the following compounds are soluble? Naoh sodium hydroxide no 5. Rbno 3 rubidium nitrate soluble 6. Pbco 3 =c.bso 3 =d.zinc hydroxide =e.sodium acetate =f.silver iodide =g.cadmium (ii) sulfide =h.zinc carbonate =. Naoh sodium hydroxide soluble 5. Classify each of the substances as being soluble or insoluble in water. Chemical formula name solubility 1. Cs 2so 4 cesium sulfate soluble 7. They determine if the ionic compound is soluble or insoluble using the rules, determine which rule(s) apply, and write the formula. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Some of the worksheets for this concept are solubility rules work answers, chemical formula name solubility, webassign, solubility rules, solubility rules and common ions, chapter 4. Agno3 and ag (c2h3o2) are common soluble salts of silver; Agcn with ksp = 2.0 × 10 ‐ 12. Ag2so4, pbso4, hg2so4, caso4, srso4, and baso4. Baso4 with ksp = 1.5 × 10 ‐ 9. Rbno 3 rubidium nitrate soluble 6. Name or give the chemical formula for each of the following compounds. Salts containing the ammonium ion (nh 4 +) are. Rbno3 rubidium nitrate yes 6. The following are the solubility rules for common ionic solids. State whether they are soluble (will dissolve) or insoluble (will not dissolve) in solution. Web solubility rules worksheet classify each of the substances as being soluble or insoluble in water. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Classify each of the substances as being soluble or insoluble in water. Web solubility rules worksheet as you work through the steps in the lab procedures, record your experimental values and the results on this worksheet. State. It can be helpful to write out the empirical formula so you can identify the ions that make up the compound. Copper (ii) sulfide = i. All chlorides are soluble except: In general, are compounds containing ammonium ions or ions from group 1 on the periodic table soluble or insoluble? Some of the worksheets for this concept are solubility rules. Chemical formula name solubility 1. Cs 2so 4 cesium sulfate soluble 7. In general, are compounds containing ammonium ions or ions from group 1 on the periodic table soluble or insoluble? Mg(oh)2 with ksp = 9.0 × 10 ‐ 12. Fes with ksp = 3.7 × 10 ‐ 19. Nh 4ch 3coo ammonium acetate soluble 2. Most silver salts are insoluble. Ag2so4, pbso4, hg2so4, caso4, srso4, and baso4. Web use the chart above to answer the following questions about solubility. Investigating trends in solubility additional observations: Web $4.00 4.8 (21) pdf add one to cart wish list solubility rules handout and fill in the blank worksheet created by powerup learning memorizing solubility rules will improve your students’ ability to accurately predict products in a chemical reaction. Pbco 3 =c.bso 3 =d.zinc hydroxide =e.sodium acetate =f.silver iodide =g.cadmium (ii) sulfide =h.zinc carbonate =. Web solubility rules all. Virtually all others are insoluble. Therefore, na 2 so 4 and srcl 2 are both soluble. Web use the chart above to answer the following questions about solubility. Agno3 and ag (c2h3o2) are common soluble salts of silver; Nh 4ch 3coo ammonium acetate soluble 2. It can be helpful to write out the empirical formula so you can identify the ions that make up the compound. They determine if the ionic compound is soluble or insoluble using the rules, determine which rule (s) apply, and write the formula. Web solubility rules worksheet classify each of the substances as being soluble or insoluble in water. Nh4c2h3o2. Which of the following compounds are soluble? Ag2so4, pbso4, hg2so4, caso4, srso4, and baso4. Web solubility rules a solute is considered soluble if an appreciable amount of it can be dissolved in a given amount of the solvent. If there two rules appear to contradict each other, the preceding rule takes precedence. Mg(oh)2 with ksp = 9.0 × 10 ‐ 12. Identify the compound whose solubility you want to check. Some of the worksheets for this concept are solubility rules work answers, chemical formula name solubility, webassign, solubility rules, solubility rules and common ions, chapter 4 work 3 molecular net ionic equations, work solubility graphs name, solubility curves work. Agcn with ksp = 2.0 × 10 ‐ 12. Web calculate the solubility in moles/l of each of three salts and the concentration of the cations in mg/ml in each of the saturated solutions. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Look up each ion in the solubility rules. Chemical formula name solubility 1. Chemical formula name solubility 1. The following are the solubility rules for common ionic solids. Classify each of the substances as being soluble or insoluble in water. Ba(oh) 2 barium hydroxide soluble 3. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Web solubility rules worksheet classify each of the substances as being soluble or insoluble in water. State whether they are soluble (will dissolve) or insoluble (will not dissolve) in solution. Rbno3 rubidium nitrate yes 6. They determine if the ionic compound is soluble or insoluble using the rules, determine which rule (s) apply, and write the formula. The table below shows the structural formulas for three different compounds. The following are the solubility rules for common ionic solids. Mg(oh)2 with ksp = 9.0 × 10 ‐ 12. Ag2so4, pbso4, hg2so4, caso4, srso4, and baso4. Web 5 name or give the chemical formula for each of the following compounds. Web use the chart above to answer the following questions about solubility. Some of the worksheets for this concept are solubility rules work answers, chemical formula name solubility, webassign, solubility rules, solubility rules and common ions, chapter 4 work 3 molecular net ionic equations, work solubility graphs name, solubility curves work answers. Feco3 iron (ii) carbonate yes 4. The following reactions take place in water. Web it includes teacher instructions, a student handout for 5 solubility rules, a student worksheet, and a key. Investigating trends in solubility additional observations: Fes with ksp = 3.7 × 10 ‐ 19. Web the solubility rules say that all ionic sodium compounds are soluble and all ionic chloride compounds are soluble, except for ag +, hg 2 2+, and pb 2+, which are not being considered here. Look up each ion in the solubility rules. Salts containing the ammonium ion (nh 4 +) are.Solubility Worksheet With Answers
Unit VIB Solubility Rules Worksheet
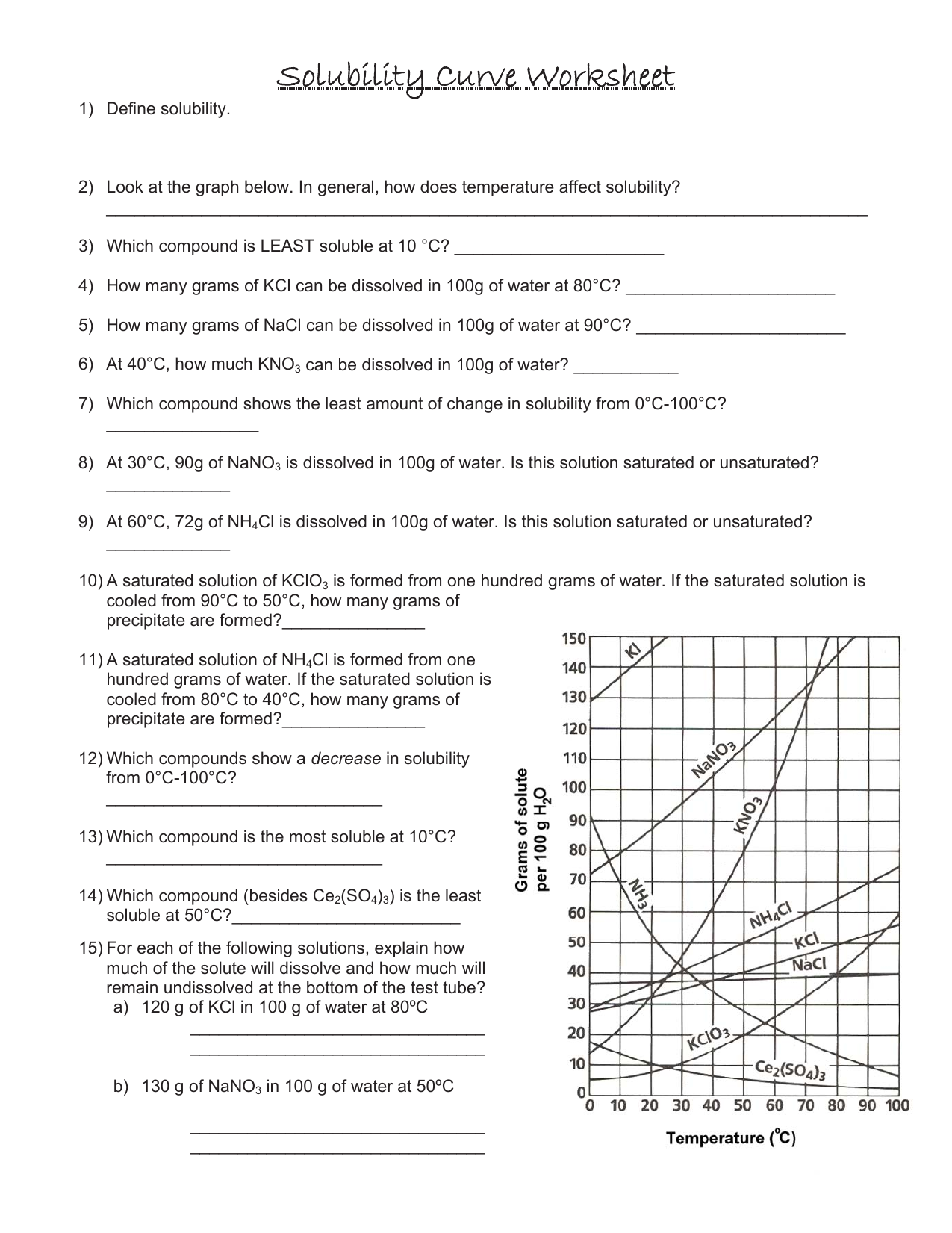
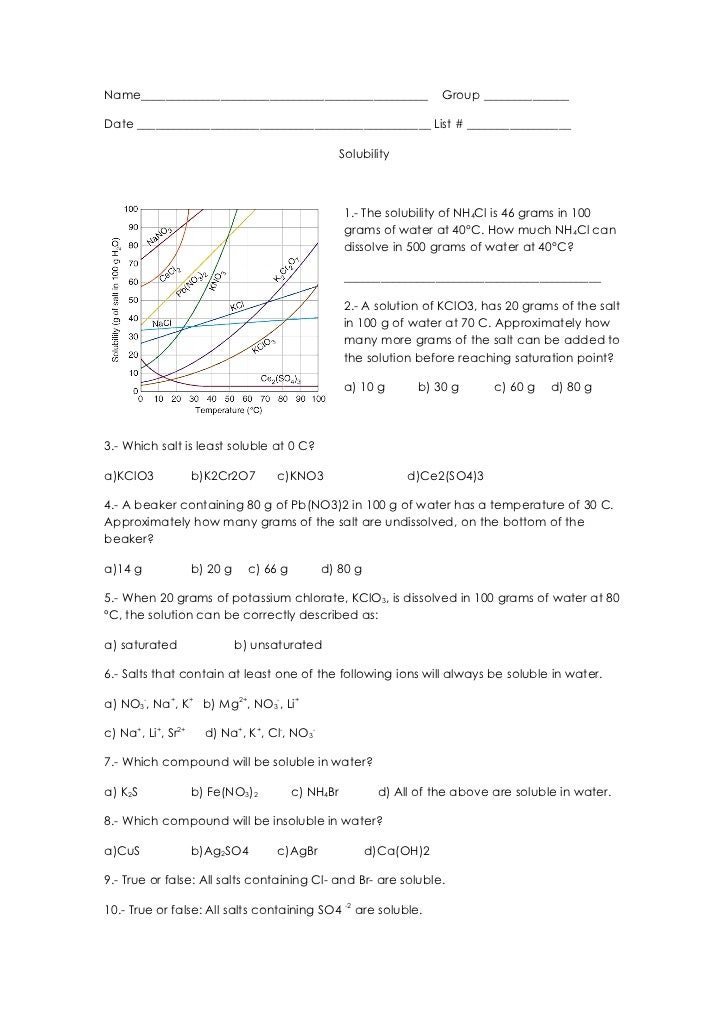
Solubility Curve Practice Worksheet Answers Solubility Graph
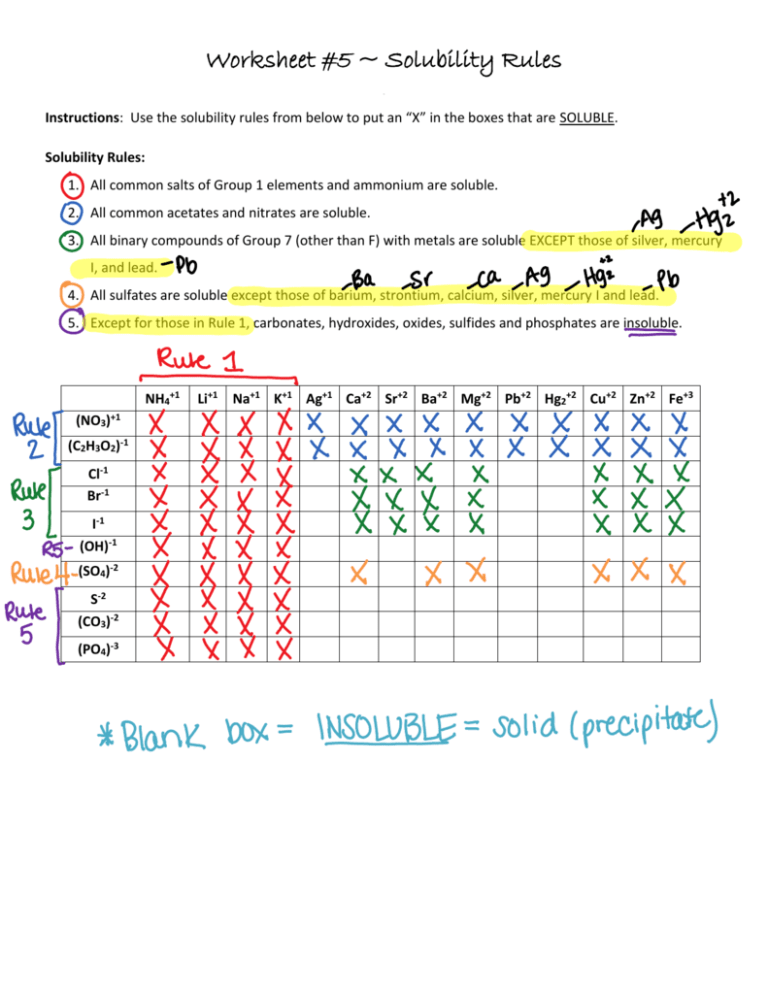
Solubility Rules Worksheet KEY
solubility rules worksheet answers
Solubility Rules Practice Worksheet
Solubility rules worksheet Answers StuDocu
worksheet. Solubility Rules Worksheet. Grass Fedjp Worksheet Study Site
Solubility Rules Worksheet With Answers
Solubility Rules Lab
Feco 3 Iron (Ii) Carbonate Insoluble 4.
State Whether They Are Soluble (Will Dissolve) Or Insoluble (Will Not Dissolve) In Solution.
Web Solubility Rules Worksheet As You Work Through The Steps In The Lab Procedures, Record Your Experimental Values And The Results On This Worksheet.
Chemical Formula Name Solubility 1.
Related Post: