Specific Heat Worksheet 1
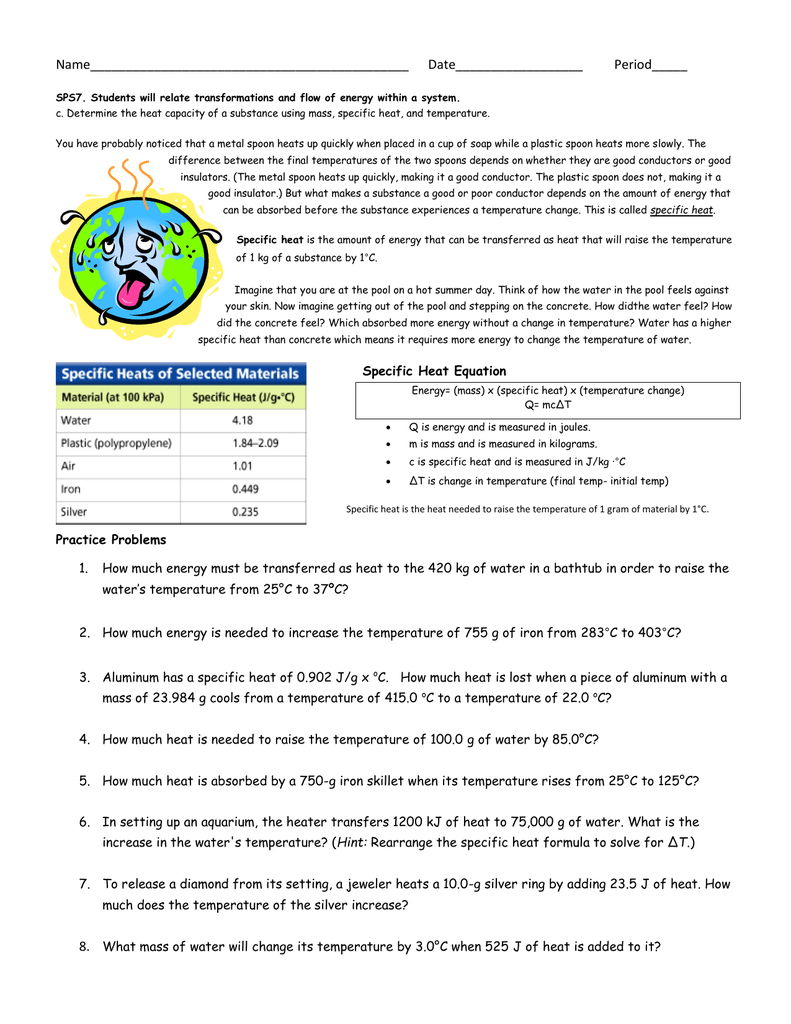
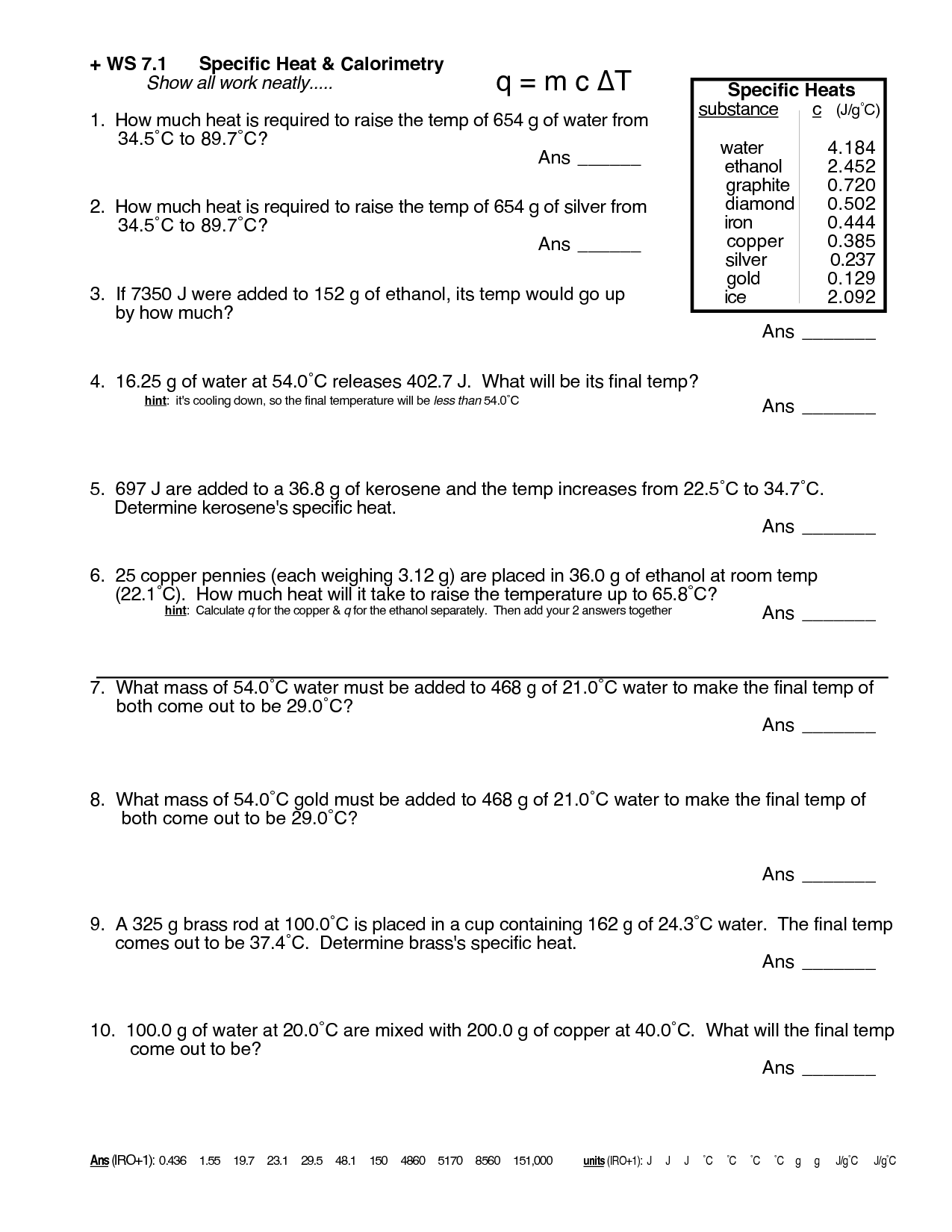
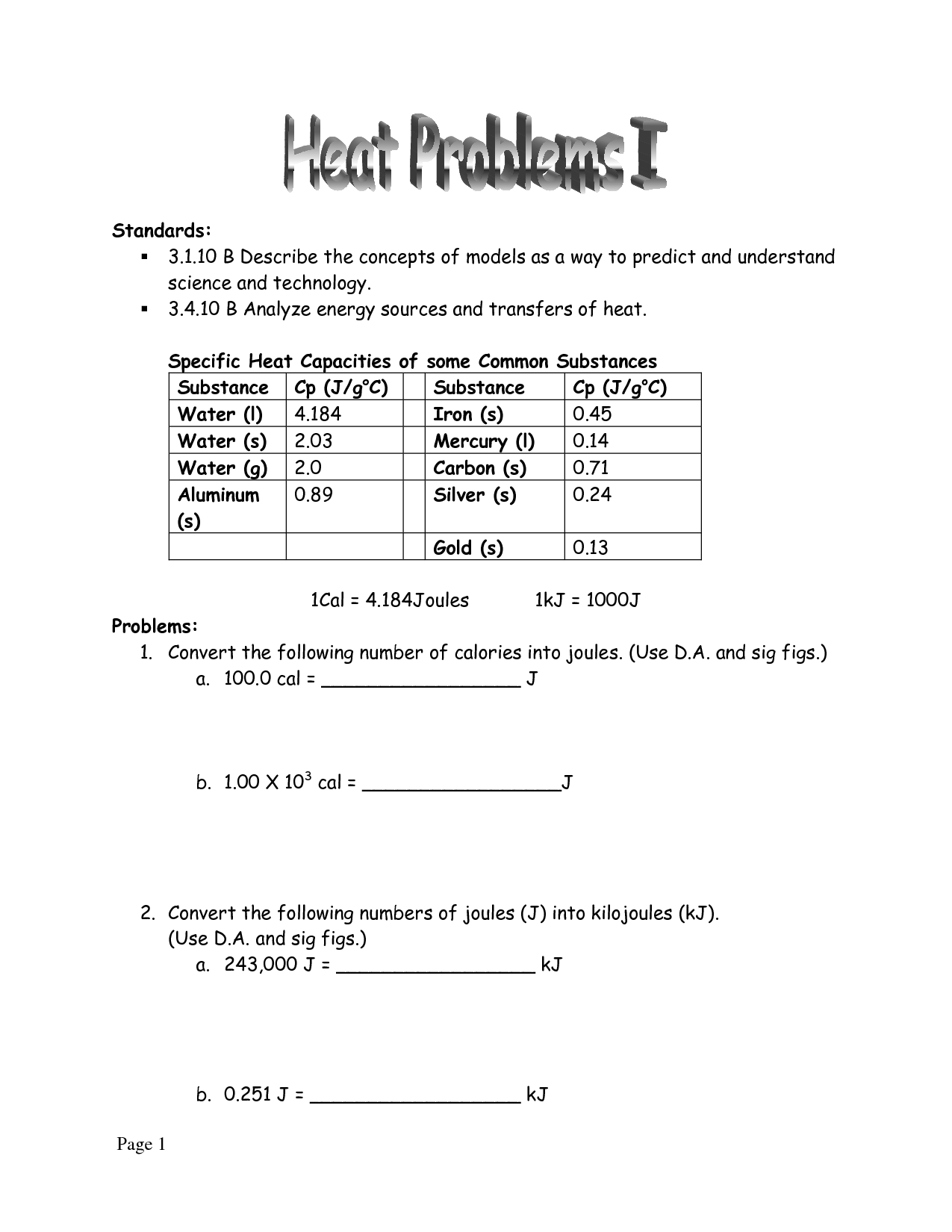
Specific Heat Worksheet 1 - This worksheet is designed for gcse physics students. Water 4.179 aluminum 0.900 copper 0.385 iron 0.450 granite 0.790. Heat is not the same as temperature, yet they are related. Caloric and joule’s discovery questions: How much heat in kilojoules is released when 25.0 g of water is cooled from 85.0ºc to 40.0ºc? How much heat is needed to raise a 0.30 kg piece of aluminum from 30. Explain what the specific heat of an object means/tells us? When heated, the temperature of a water sample increased from 15°c to 39°c. The water has a mass of 500g, a specific heat of 4.184 (j/gx0c), and had a change in temperature of 22 0c. If the temperature of 100 g of water increases from 10 to 45 c, how much heat is absorbed? The specific heat of aluminum is 0.901 j/(g · oc). Each question has been carefully designed to range in difficulty from simple calculations. B) 1.96 kj of heat are added to 500. So, we can now compare the specific heat capacity of a substance on a per gram bases. 5.0 g of copper was heated from 20°c to 80°c. Web specific heat and heat capacity worksheet. Explain how they differ from each other. Use q = (m)(cp))(δt) to solve the following problems. A metal weighing 50.0 g absorbs 220.0 j of heat when its temperature increases by 120.0°c. Suppose the skillet is cooled to room temperature, 23.9 oc. It includes a series of questions of increasing challenge, with answers and extra supporting videos available at the link on the bottom of each page or via the qr code. Specific latent heat fill in the blanks: Click the card to flip 👆. This worksheet is designed for gcse physics students. Specific heat, latent heat, phase change graphs, and calorimetry. What was the caloric model? The water has a mass of 500g, a specific heat of 4.184 (j/gx0c), and had a change in temperature of 22 0c. Q = mc δ t, c = q ( j) m ( g) δ t ( k) How did it fail to explain the heating of drill bits when they got dull? Show. How much heat in kilojoules is released when 25.0 g of water is cooled from 85.0ºc to 40.0ºc? B) 1.96 kj of heat are added to 500. An aluminum skillet weighing 1.58 kg is heated on a stove to 173 oc. Web what is the specific heat? Web the specific heat capacity is the amount of heat it takes to. How much heat energy (joules) must be removed to cause this cooling? Web specific heat worksheet 1. Web what is the specific heat? B) 1.96 kj of heat are added to 500. 100.5 j 2.01 j/g 2 a metal with a specific heat of 0.780 j/g c requires 45.0 j of heat to raise the temperature by 2.00 c. Click the card to flip 👆. The water has a mass of 500g, a specific heat of 4.184 (j/gx0c), and had a change in temperature of 22 0c. It absorbed 4300 joules of heat. This worksheet is designed for gcse physics students. The table to the right list the specific heat (heat capacity) of four common materials aluminum, beryllium, gold,. A) 10.0 kg of water loses 232 kj of heat. Q=mcδt, where q = heat energy, m = mass, and t = temperature. How is knowing the specific heat of an object useful? How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to 55°c, if the specific heat of aluminum is. Calculate the heat capacity of iron. Worksheet add to my workbooks (7) download file pdf embed in my website or blog add to google classroom add to. This value also depends on the nature of the chemical bonds in the substance, and its phase. How did it fail to explain the heating of drill bits when they got dull? A). This worksheet is designed for gcse physics students. Suppose the skillet is cooled to room temperature, 23.9 oc. Use q = (m)(δt)(cp) to solve the following problems. Web specific heat and heat capacity worksheet. Education.com has been visited by 100k+ users in the past month How much heat is needed to raise a 0.30 kg piece of aluminum from 30. What is the specific heat of the metal? An aluminum skillet weighing 1.58 kg is heated on a stove to 173 oc. Show all work and units. A) 10.0 kg of water loses 232 kj of heat. Determine if it’s endothermic or exothermic 1. Web live worksheets > english > science > heat > specific latent heat. This worksheet is designed for gcse physics students. When heated, the temperature of a water sample increased from 15°c to 39°c. Show all work and units. Web specific heat and heat capacity worksheet. Web the table below shows the specific heats of some common substances. Specific heat, latent heat, phase change graphs, and calorimetry objective a: Water 4.179 aluminum 0.900 copper 0.385 iron 0.450 granite 0.790. Q=mcδt, where q = heat energy, m = mass, and t = temperature. (q= m c δ t) b. Calculate the specific heat capacity of iron. Use q = (m)(δt)(cp) to solve the following problems. A metal weighing 50.0 g absorbs 220.0 j of heat when its temperature increases by 120.0°c. What is the mass of the metal? Use the table below to answer the following questions. 1 find the amount of heat (q) needed to raise the temperature of 5.00 g of a substance from 20.0qc to 30.0qc if the specific heat of the substance is 2.01 j/gqc.100.5 j 2 a metal with a specific heat of 0.780 j/gqc. How did it fail to explain the heating of drill bits when they got dull? It includes a series of questions of increasing challenge, with answers and extra supporting videos available at the link on the bottom of each page or via the qr code. Web what is the specific heat? Education.com has been visited by 100k+ users in the past month When 3.0 kg of water is cooled from 80.0(c to 10.0(c, how much heat energy is lost? 5.0 g of copper was heated from 20°c to 80°c. For q= m c δ t : Q = mc t questions: Specific latent heat fill in the blanks: Show all work and proper units. A) 10.0 kg of water loses 232 kj of heat. Use q = (m)(cp))(δt) to solve the following problems. Caloric and joule’s discovery questions: How much heat is needed to raise a 0.30 kg piece of aluminum from 30.(c to 150(c?Worksheet Introduction to Specific Heat Capacities
Specific heat capacity interactive worksheet
Calculating Specific Heat Worksheet Answers Yooob —
Specific Heat Equation Practice Worksheet
Other Worksheet Category Page 375
Heat Transfer/ Specific Heat Problems Worksheet
10 Best Images of Science Worksheets On Heat Temperature Science
Specific Heat Worksheet Answers
Calculating Specific Heat Worksheet
Specific Heat Capacity Worksheet. worksheet
The Specific Heat Of Aluminum Is 0.901 J/(G · Oc).
Web Specific Heat Capacity Worksheet.
So, We Can Now Compare The Specific Heat Capacity Of A Substance On A Per Gram Bases.
Worksheet Add To My Workbooks (7) Download File Pdf Embed In My Website Or Blog Add To Google Classroom Add To.
Related Post: