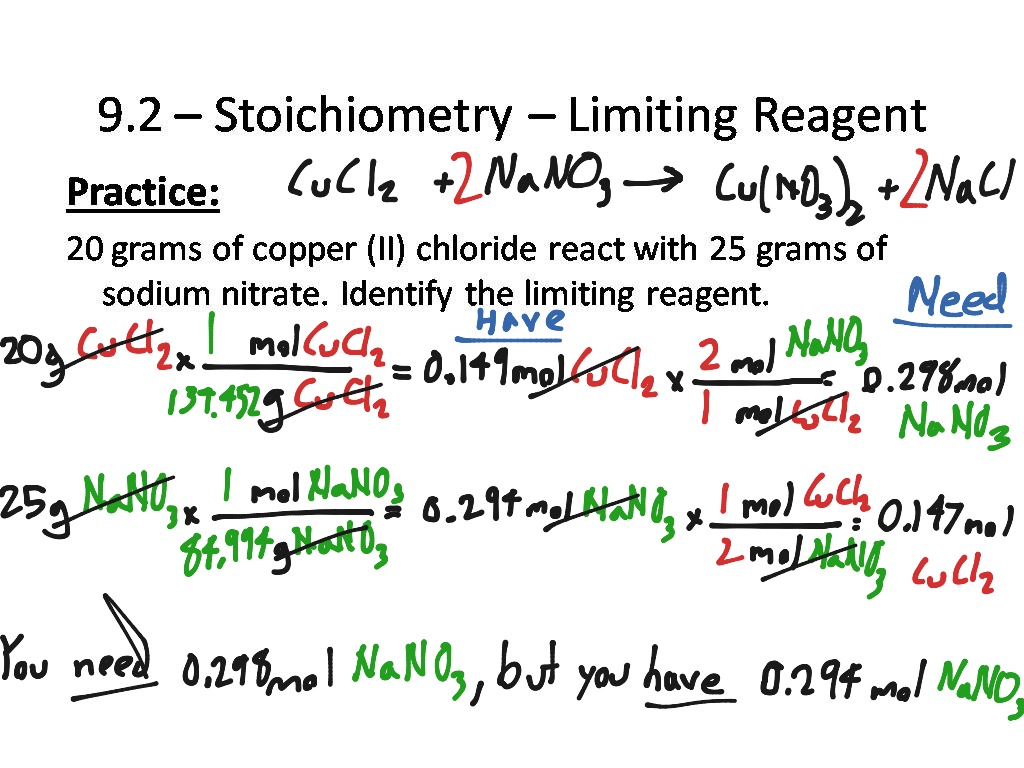Stoichiometry Limiting Reagent Worksheet
Stoichiometry Limiting Reagent Worksheet - Web answer limiting reagent problem strategies: It will be based on the mass of the reactants present, and on the stoichiometry of the reaction. Included in the chemistry instructor resources subscription. To determine the grams of excess reagent, subtract the amount you need from the amount that you have, then using the molar mass, convert the moles left to grams. .the solutions are on the back wall. If given mass, divide by formula weight to convert to moles (this is the mass to mole step from the section 4.1,3. Web stoichiometry & limiting reagents quiz. Determine the mass of iodine i2, which could be produced? Web given the equation below, determine the limiting reactant, and calculate how many grams of cu can be formed from the reaction of 18.1 g of nh3 and 90.4 g of cuo. (unbalanced) al 2 (so 3) 3 + naoh na 2 so 3 + al(oh) 3 5) if 10.0 g of al 2 (so 3) 3 is reacted with 10.0 g of naoh, determine the limiting reagent and the excess reagent 6) determine the number of moles of al(oh) 3 produced 7) determine the number of grams. Web given the equation below, determine the limiting reactant, and calculate how many grams of cu can be formed from the reaction of 18.1 g of nh3 and 90.4 g of cuo. Suppose 13.7 g of c2h2 reacts with 18.5 g o2 according to the reaction below. 4) compare what you have to what you need. 3) based on the. .the solutions are on the back wall. Web this worksheet has 10 limiting reactant stoichiometry problems. Web answer limiting reagent problem strategies: The worksheet doesn't specify a product, so they can convert the reactants to either product and compare. Included in the chemistry instructor resources subscription. The first worksheet is composed of 10 stoichiometry problems. 3) based on the moles that you have, calculate the moles that you need of the other reagent to react with each of those amounts. Web answer limiting reagent problem strategies: Determine the mass of lithium hydroxide 8. Limiting reagents (worksheet) solution q1. If 6.80 g of ph 3 and 6.80 g of o 2 are combined according to the (unbalanced) reaction shown below, __ ph 3 + __ o 2 → __ p 4o 10 + __ h 2o which is the. Determine the mass of lithium hydroxide 8. It will be based on the mass of the reactants present, and on. Nitrogen gas can react with hydrogen gas to form gaseous ammonia. 2no(g) + o2 ( 2no2 in one experiment 0.866 mol of no is mixed with 0.503 mol of o2. The worksheet doesn't specify a product, so they can convert the reactants to either product and compare. Gravimetric analysis and precipitation gravimetry. Web stoichiometry & limiting reagents quiz. (unbalanced) al 2 (so 3) 3 + naoh na 2 so 3 + al(oh) 3 5) if 10.0 g of al 2 (so 3) 3 is reacted with 10.0 g of naoh, determine the limiting reagent and the excess reagent 6) determine the number of moles of al(oh) 3 produced 7) determine the number of grams. The first worksheet is. Web stoichiometry/limiting reagent practice ap chemistry (practice, practice, practice. Web the principles of stoichiometry and limiting reagents will be used to predict the amount of product that should be produced when mixing two solutions to produce an insoluble product. An example of the type of problem is:aluminum sulfate and barium chlorid 2 nh3 (g) + 3 cuo (s) n2 (g). This equation is already balanced. When 50.0 g of mgco3 react completely with h3po4, as shown below,15.8 g of co2 is produced. I) what mass of iodine was produced? Balance the equation first) q2. This online quiz is intended to give you extra practice in performing stoichiometric conversions, including limiting reagent and percent yield problems. 1) write the balanced equation for the reaction of lead (ii) nitrate with sodium iodide to form sodium nitrate and lead (ii) iodide: Web answer limiting reagent problem strategies: Web stoichiometry & limiting reagents quiz. What is the mass of co2 produced? If 6.80 g of ph 3 and 6.80 g of o 2 are combined according to the (unbalanced). 2015 ap chemistry free response 2a (part 1 of 2) Web stoichiometry/limiting reagent practice ap chemistry (practice, practice, practice. This online quiz is intended to give you extra practice in performing stoichiometric conversions, including limiting reagent and percent yield problems. To determine the amounts of product (either grams or moles), you must start with the limiting reagent. B) if, in. Web how many grams of lithium nitrate will be needed to make 250 grams of lithium sulfate, assuming that you have an adequate amount of lead (iv) sulfate to do the reaction? Web 1) make sure the equation is balanced. Determine the mass of iodine i2, which could be produced? This quiz aligns with the following ngss standard (s): Calculating the amount of product formed from a limiting reactant. Gravimetric analysis and precipitation gravimetry. Limiting reactant and reaction yields. Nitrogen gas can react with hydrogen gas to form gaseous ammonia. To determine the amounts of product (either grams or moles), you must start with the limiting reagent. .show all work (including balanced equations). This equation is already balanced. Web understand the concept of the limiting reagent success criteria convert between numbers of atoms, moles, and mass of sample by using avogadro’s number and the appropriate molar mass calculate the empirical formula of a compound from percent composition data Pb(no 3) 2 + 2 nai → pbi 2 + 2 nano 3. G lino3 = 250 g li2so4 mol li2so4 mol lino3 g lino3 Balance the equation first) q2. Divide moles of each reactant by it's stoichiometric coefficient. 1) write the balanced equation for the reaction of lead (ii) nitrate with sodium iodide to form sodium nitrate and lead (ii) iodide: If 6.80 g of ph 3 and 6.80 g of o 2 are combined according to the (unbalanced) reaction shown below, __ ph 3 + __ o 2 → __ p 4o 10 + __ h 2o which is the. Web limiting reagents movie text earlier, we were working with the reaction: When 50.0 g of mgco3 react completely with h3po4, as shown below,15.8 g of co2 is produced. Web this worksheet has 10 limiting reactant stoichiometry problems. Calculating the amount of product formed from a limiting reactant. 4ko2(s) + 2h2o(l) → 4koh(s) + 3o2(g) 2. Web this worksheet gives them two measurements. (unbalanced) al 2 (so 3) 3 + naoh na 2 so 3 + al(oh) 3 5) if 10.0 g of al 2 (so 3) 3 is reacted with 10.0 g of naoh, determine the limiting reagent and the excess reagent 6) determine the number of moles of al(oh) 3 produced 7) determine the number of grams. Students need to determine the limiting reactant in each process. If given mass, divide by formula weight to convert to moles (this is the mass to mole step from the section 4.1,3. Web this reagent is the lr. What is the mass of co2 produced? G lino3 = 250 g li2so4 mol li2so4 mol lino3 g lino3 Web what is the limiting reagent? Web stoichiometry & limiting reagents quiz. Web stoichiometry/limiting reagent practice ap chemistry (practice, practice, practice. Pb (no3)2 (aq) + 2 nai (aq) pbi2 (s) + 2 nano3 (aq) 2) if i start with 25.0. The worksheet doesn't specify a product, so they can convert the reactants to either product and compare. Pb(no 3) 2 + 2 nai → pbi 2 + 2 nano 3.Stoichiometry Limiting Reagent Worksheet Instructional Fair canvas
Limiting Reagent Worksheet Answer Key Kayra Excel
Stoichiometry Limiting Reagent Worksheet Melaniea Marobrasil
Limiting Reagent Worksheet 2
Limiting Reagent Worksheet Answers Briefencounters
Stoichiometry Limiting Reagent Worksheet
Stoichiometry Limiting Reagent Worksheet
Stoichiometry Limiting Reagent Worksheet Answers —
Stoichiometry Limiting Reagent Worksheet
Stoichiometry Limiting Reagent Worksheet Answers —
.The Solutions Are On The Back Wall.
2015 Ap Chemistry Free Response 2A (Part 1 Of 2)
2 Nh3 (G) + 3 Cuo (S) N2 (G) + 3 Cu (S) + 3 H2O (G) Ans:
Nitrogen Gas Can React With Hydrogen Gas To Form Gaseous Ammonia.
Related Post: