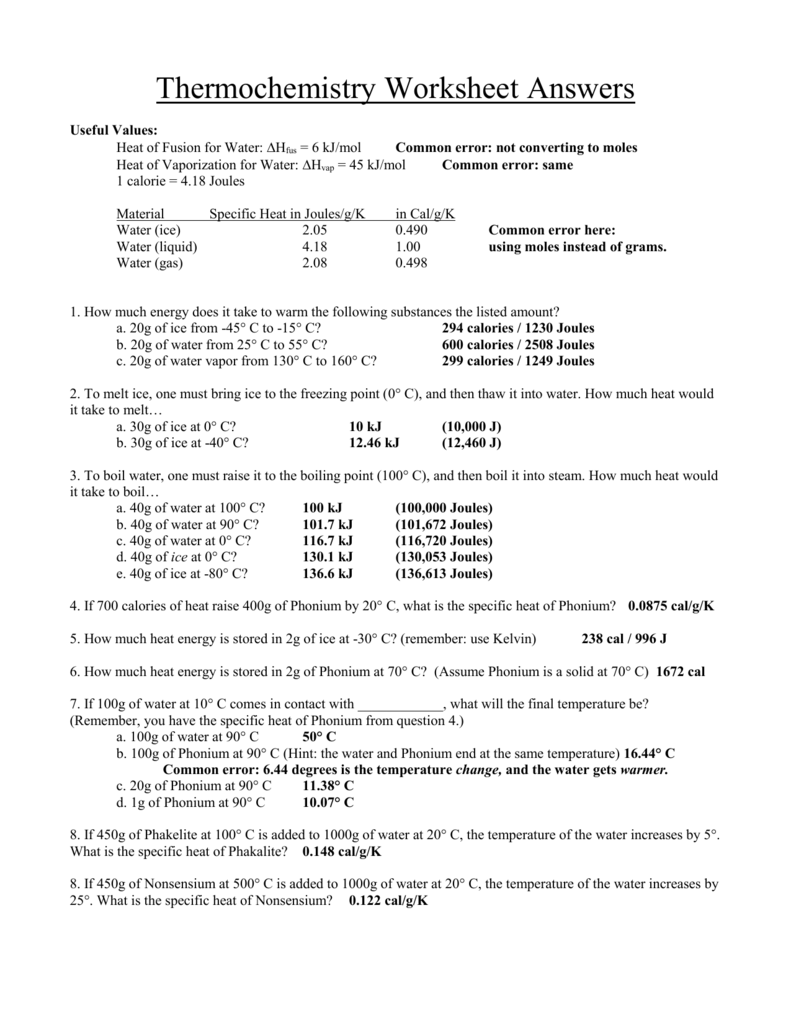
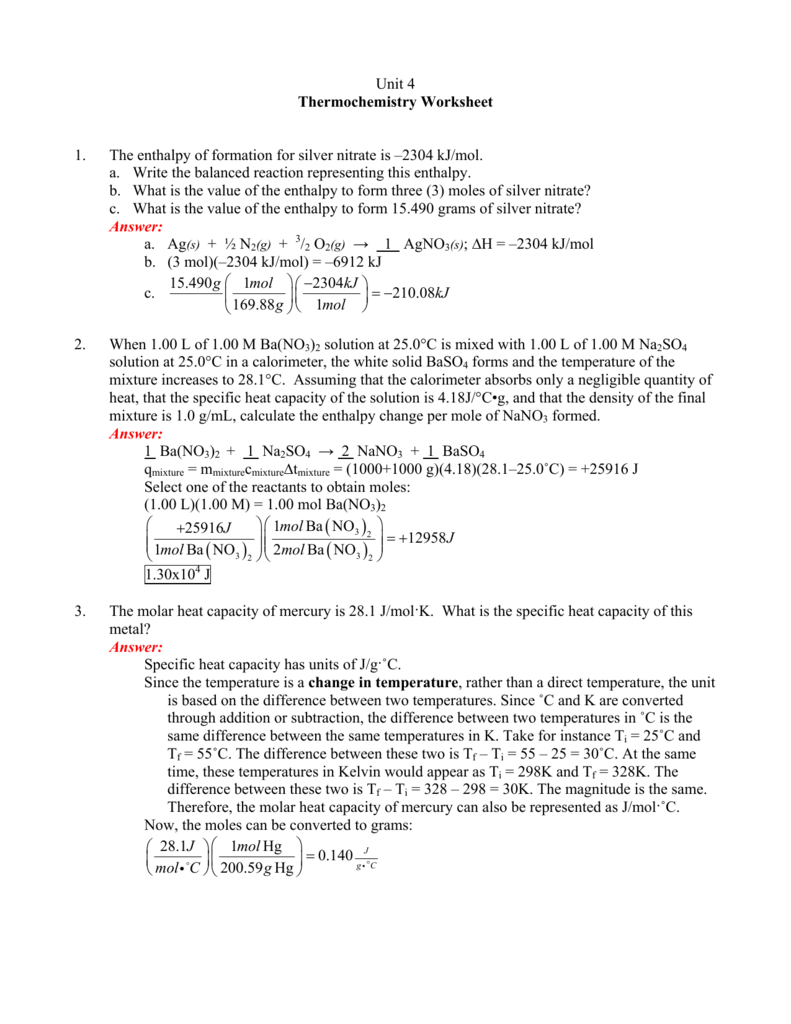
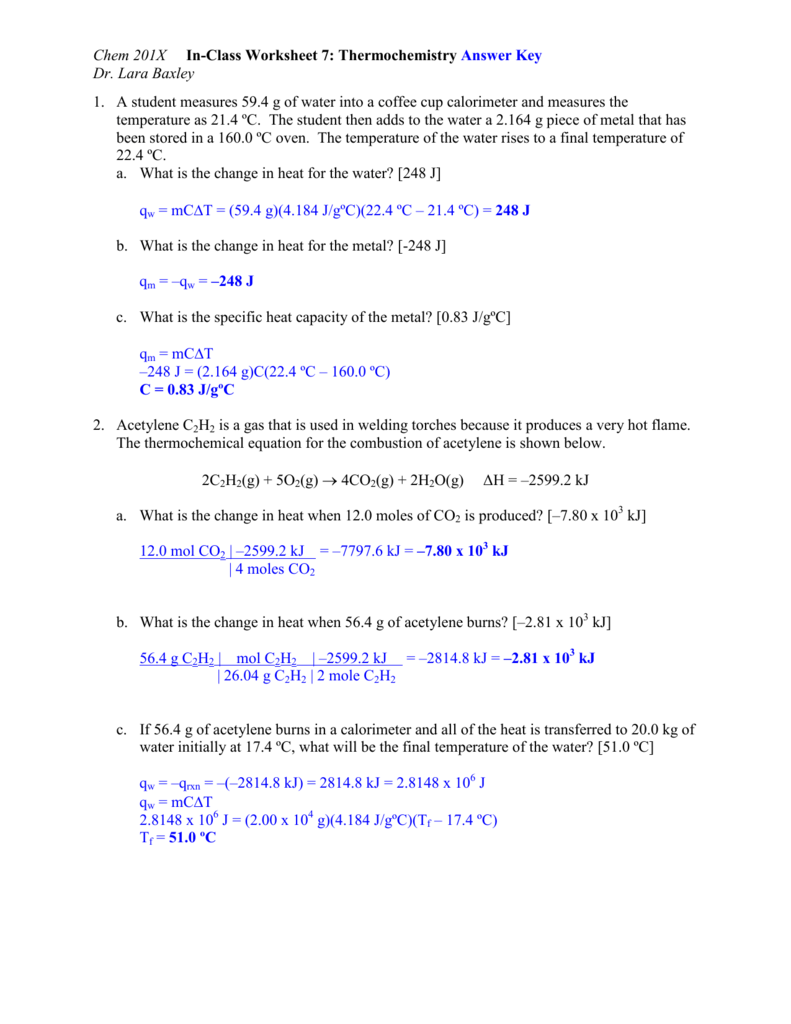
Thermochemistry Worksheet Answer Key
Thermochemistry Worksheet Answer Key - ( c of al = 2.42 j/g * k) q2 a 27.7 g sample of ethylene glycol loses 688 j of heat. Web georgetown independent school district / gisd home The following describes the reaction that takes place when a typical fat, glyceryl trioleate, is metabolized by the body: Two ways that the internal energy of an object can be categorized are potential and kinetic energy. Heat lost by system to surroundings is _________________. How much heat energy must be expelled by the body to rid the body of one pound of fat. Web thermochemistry practice worksheet answer key. Is this reaction exothermic or endothermic? Web thermochemistry worksheet key 1. Web the five solutions are: Some of the worksheets for this concept are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work 1, ap chemistry review work unit 4, answers thermochemistry practice problems. This is the same answer you obtained in q1, as expected on the basis of hess’s law. 2s 3o 2 2so 3 h 7914kj is this endothermic or exothermic 2. What is. But notice that the answer we obtain here is the following sum, where δ hof (h 2) = 0, because h 2 ( g) is an element in its standard state: Fill in the blanks heat gained (absorbed) is considered _____________; What is the final temperature of the part? How much heat energy must be expelled by the body to. What is the heat change when 472 g of carbon reacts with excess o 2 according to the following equation. Gibbs free energy second law of thermodynamics spontaneity at different temperatures electrochemistry > Use heat of formation values. Calculate the heat of the reaction for the following reaction: Of water at 90.0 oc is added to 100.0 ml of water. Web the five solutions are: (explain what happens in terms of heat flow, and what happens to the temperature of the surroundings) 2. Potential energy centers on stored energy due to its position and chemical makeup. What was the initial temperature of the ethylene glycol if the final temperature is 32.5°c ( c of ethylene glycol = 2.42 j/g *k). How much heat energy must be expelled by the body to rid the body of one pound of fat. Web georgetown independent school district / gisd home This is the same answer you obtained in q1, as expected on the basis of hess’s law. Give at least 3 examples of each that you encounter in everyday life. Use heat of. How much heat energy must be expelled by the body to rid the body of one pound of fat. Web thermochemistry worksheet key 1. What is the heat change when 472 g of carbon reacts with excess o 2 according to the following equation. 1) (50.0 g) (15 °c) (2.06 j/g °c) 2) (50.0 g / 18.0 g/mol) (6.02 kj/mol). Web up to 24% cash back the temperature of the solution goes up how many degrees? Heat work temperature exothermic endothermic first law of thermodynamics system surroundings kinetic energy potential energy q2: Kinetic energy centers on energy an object has while in motion. Web thermochemistry worksheet key 1. Web the five solutions are: Calculate the heat of the reaction for the following reaction: Answer all of the questions. Save or instantly send your ready documents. Kinetic energy centers on energy an object has while in motion. The following describes the reaction that takes place when a typical fat, glyceryl trioleate, is metabolized by the body: Some of the worksheets for this concept are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work 1, ap chemistry review work unit 4, answers thermochemistry practice problems. Kinetic energy centers on energy an object has while in motion. Potential energy centers on stored energy due to its position and chemical makeup. Web q1 a 295 g aluminum engine part. Definition define the following terms: Web thermochemistry worksheet key 1. Web georgetown independent school district / gisd home Web thermochemistry answer key 1. The following describes the reaction that takes place when a typical fat, glyceryl trioleate, is metabolized by the body: Easily fill out pdf blank, edit, and sign them. 1) (50.0 g) (15 °c) (2.06 j/g °c) 2) (50.0 g / 18.0 g/mol) (6.02 kj/mol) 3) (50.0 g) (100 °c) (4.184 j/g °c) 4) (50.0 g / 18.0 g/mol) (40.7 kj/mol) 5) (50.0 g) (20 °c) (2.02 j/g °c) 10. Use heat of formation values. Web complete unit thermochemistry intro and joule conversions wksh 1 answer key online with us legal forms. Definition define the following terms: The following describes the reaction that takes place when a typical fat, glyceryl trioleate, is metabolized by the body: The following describes the reaction that takes place when a typical fat, glyceryl trioleate, is metabolized by the body: Give at least 3 examples of each that you encounter in everyday life. Potential energy centers on stored energy due to its position and chemical makeup. 2f2(g) + 2h2o(l) ( 4hf(g) + o2(g) (h = ? Answer all of the questions. Calculate the heat of the reaction for the following reaction: Distinguish between endothermic and exothermic processes. Web name _____ date _____ period _____ thermochemistry worksheet chemistry; Heat work temperature exothermic endothermic first law of thermodynamics system surroundings kinetic energy potential energy q2: How much heat energy must be expelled by the body to rid the body of one pound of fat. Gibbs free energy second law of thermodynamics spontaneity at different temperatures electrochemistry > Web thermochemistry answer key 1. So let's start by calling the final, ending temperature 'x.' keep in mind that both the water and the lead will wind up at the temperature we are calling 'x.' Some of the worksheets for this concept are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work 1, ap chemistry review work unit 4, answers thermochemistry practice problems. Is this reaction exothermic or endothermic? What is an endothermic reaction? The following describes the reaction that takes place when a typical fat, glyceryl trioleate, is metabolized by the body: Web georgetown independent school district / gisd home (explain what happens in terms of heat flow, and what happens to the temperature of the surroundings) 2. Of water at 90.0 oc is added to 100.0 ml of water at 30.0 oc? What is the heat change when 472 g of carbon reacts with excess o 2 according to the following equation. Fill in the blanks heat gained (absorbed) is considered _____________; Use heat of formation values. 20) what is the final temperature when 150.0 ml. So let's start by calling the final, ending temperature 'x.' keep in mind that both the water and the lead will wind up at the temperature we are calling 'x.' The five number sections correspond to the following: Answer all of the questions. Heat work temperature exothermic endothermic first law of thermodynamics system surroundings kinetic energy potential energy q2: 2s 3o 2 2so 3 h 7914kj is this endothermic or exothermic 2. Easily fill out pdf blank, edit, and sign them.Thermochemistry Worksheet Answers
Unit 4 Thermochemistry Worksheet 1. The enthalpy of formation for
Thermochemistry Problems Worksheet Number One (answers
Thermochemistry Worksheet Answer Key Live Worksheet Online
thermochemistry worksheet 1 answers
Specific Heat Worksheet 2 Answer Key Thekidsworksheet
Thermochemistry Test Preview with Answer Key
Thermochemistry Calorimetry Worksheet Answers Reporteral
141 Thermochemistry worksheet key Thermochemistry Worksheet Key How
Worksheet Thermochemistry II Answers
What Was The Initial Temperature Of The Ethylene Glycol If The Final Temperature Is 32.5°C ( C Of Ethylene Glycol = 2.42 J/G *K)
Web Q1 A 295 G Aluminum Engine Part At An Initial Temperature Of 3.00°C Absorbs 85.0 Kj Of Heat.
But Notice That The Answer We Obtain Here Is The Following Sum, Where Δ Hof (H 2) = 0, Because H 2 ( G) Is An Element In Its Standard State:
The Following Describes The Reaction That Takes Place When A Typical Fat, Glyceryl Trioleate, Is Metabolized By The Body:
Related Post: