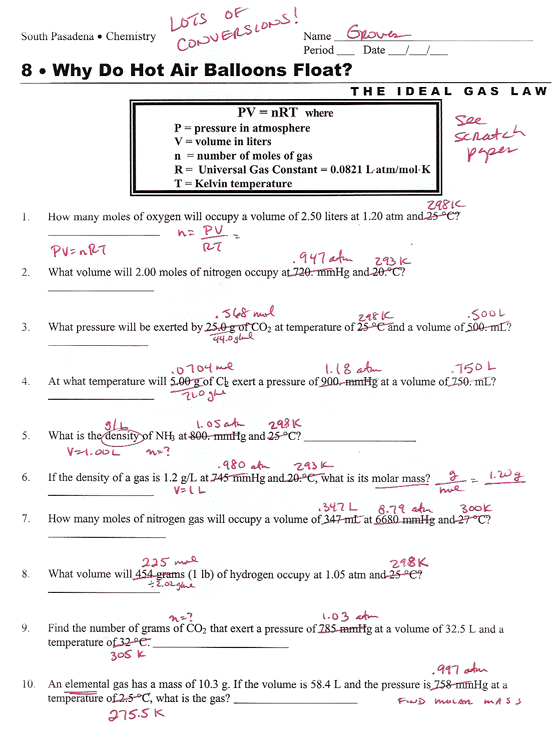
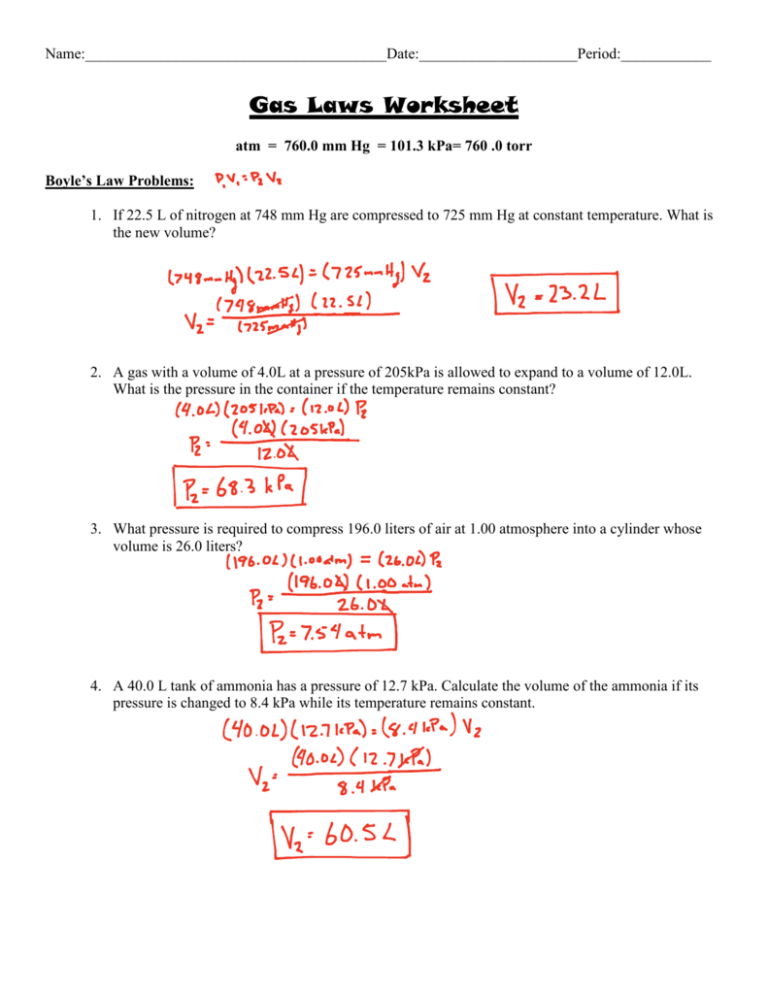
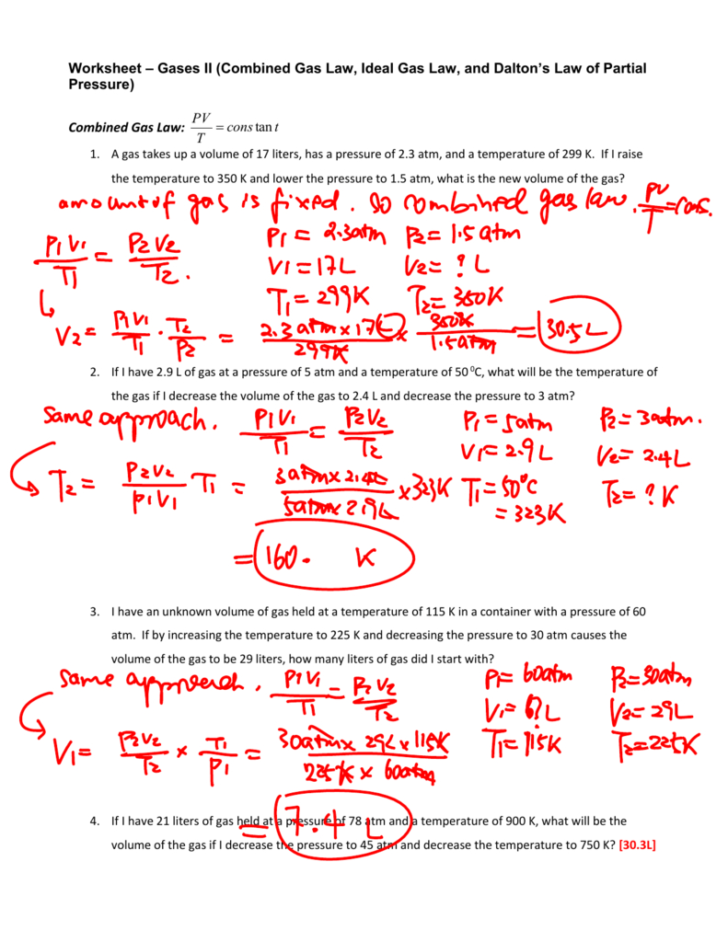
Gas Law Worksheet Answer Key
Gas Law Worksheet Answer Key - Web at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: What volume would the gas occupy at 88.2 kpa if the temperature remains constant? View full document atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle’s law problems:. Web there are many types of gas law problems, but they can generally be grouped into two main types: P t = p 1 + p 2 + p 3 +. Determine the total pressure of a gas mixture that contains oxygen at a. What is the pressure in the container if the temperature. Charles’ law, boyle’s law, and avogadro’s law (all of which will later combine into the general gas equation and ideal. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. The ideal gas law key directions: Find the gas laws worksheet answer key you want. The ideal gas law key directions: Web for students to be successful with gas laws, they must be able to convert temperature, pressure, and mole/liter units. Web at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: View full document atm. The ideal gas law key directions: Web there are many types of gas law problems, but they can generally be grouped into two main types: Determine the total pressure of a gas mixture that contains oxygen at a. View full document atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle’s law problems:. N = pv = (2.8 atm)(98. What volume would the gas occupy at 88.2 kpa if the temperature remains constant? Determine the total pressure of a gas mixture that contains oxygen at a. This worksheet will give students practice converting. Solve each of the following problems. Web the gas laws consist of three fundamental laws: What is the pressure in the container if the temperature. N = pv = (2.8 atm)(98 l) = 11. Determine the total pressure of a gas mixture that contains oxygen at a. Open it up using the cloud. Show your work, including proper units, to earn full credit. The ideal gas law key directions: Web of the three principal states of matter (gas, liquid, solid), gases show behavior that is most easily connected to molecular motion. Solve each of the following problems. Web what is the new volume? Web at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas. What is the pressure in the container if the temperature. The ideal gaslawinvestigates the relationship between pressure, volume, temperature, and moles of a gas. Web gas law worksheet #2 (dalton’s law and ideal gas law) dalton’s law: Web of the three principal states of matter (gas, liquid, solid), gases show behavior that is most easily connected to molecular motion. Find. Web 1671 k a sample of nitrogen gas has a volume of 478 cm3 and pressure of 104.1 kpa. Charles’ law, boyle’s law, and avogadro’s law (all of which will later combine into the general gas equation and ideal. Atm = 760 mm hg = 101 kpa= 760.0 torr. Open it up using the cloud. What is the pressure in. Web the gas laws consist of three fundamental laws: If 3.7 moles of propane are at a. Web there are many types of gas law problems, but they can generally be grouped into two main types: Show your work, including proper units, to earn full credit. N = pv = (2.8 atm)(98 l) = 11. P t = p 1 + p 2 + p 3 +. N = pv = (2.8 atm)(98 l) = 11. If a gas at occupies 2.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 3.50 atm? Web the gas laws consist of three fundamental laws: Determine the total pressure of a. Show your work, including proper units, to earn full credit. Web the gas laws consist of three fundamental laws: Charles’ law, boyle’s law, and avogadro’s law (all of which will later combine into the general gas equation and ideal. The ideal gas law key directions: Web at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most. What volume would the gas occupy at 88.2 kpa if the temperature remains constant? Web up to 24% cash back gas law problems gas law problems 1. Solve each of the following problems. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. Web what is the new volume? Atm = 760 mm hg = 101 kpa= 760.0 torr. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. The ideal gas law key directions: The ideal gaslawinvestigates the relationship between pressure, volume, temperature, and moles of a gas. Each quantity in the equation is usually. Web for students to be successful with gas laws, they must be able to convert temperature, pressure, and mole/liter units. Find the gas laws worksheet answer key you want. If a gas at occupies 2.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 3.50 atm? P t = p 1 + p 2 + p 3 +. Web at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Web 1671 k a sample of nitrogen gas has a volume of 478 cm3 and pressure of 104.1 kpa. This worksheet will give students practice converting. Show your work, including proper units, to earn full credit. View full document atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle’s law problems:. Determine the total pressure of a gas mixture that contains oxygen at a. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. Web the gas laws consist of three fundamental laws: Web 1671 k a sample of nitrogen gas has a volume of 478 cm3 and pressure of 104.1 kpa. What is the pressure in the container if the temperature. Show your work, including proper units, to earn full credit. Charles’ law, boyle’s law, and avogadro’s law (all of which will later combine into the general gas equation and ideal. Find the gas laws worksheet answer key you want. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. What volume would the gas occupy at 88.2 kpa if the temperature remains constant? Determine the total pressure of a gas mixture that contains oxygen at a. N = pv = (2.8 atm)(98 l) = 11. Open it up using the cloud. P t = p 1 + p 2 + p 3 +. Web there are many types of gas law problems, but they can generally be grouped into two main types: Web up to 24% cash back gas law problems gas law problems 1. This worksheet will give students practice converting.Ideal Gas Law Worksheet Answers Questions Answer Zac Sheet
Ideal Gas Law Worksheet 144 Answer Key Greenus
11 Chemistry Gas Laws Worksheet /
SOLUTION Ideal gas law worksheet 2 answer Studypool
Avogadro's Law Worksheet Answers / Solved Experiment 14 Using The Ideal
Gas Laws Worksheet Answer Key
Chemistry Gas Laws Worksheet Answer Key With Work
Gas Laws Worksheet 1
17 Mixed Gas Laws Worksheet Answers /
Gas Laws Worksheet 1 Answer Key —
If A Gas At Occupies 2.60 Liters At A Pressure Of 1.00 Atm, What Will Be Its Volume At A Pressure Of 3.50 Atm?
Web For Students To Be Successful With Gas Laws, They Must Be Able To Convert Temperature, Pressure, And Mole/Liter Units.
Solve Each Of The Following Problems.
Web Gas Law Worksheet #2 (Dalton’s Law And Ideal Gas Law) Dalton’s Law:
Related Post: